MEDGEO_2012_Presentation
advertisement

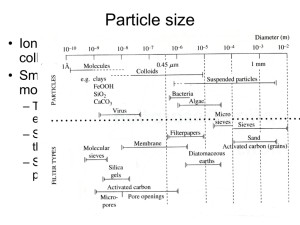
MEDGEO 2013 Conference CHARACTERIZATION OF SORPTION AND QUANTITATIVE ANALYSIS OF HYDROXYL COMPLEXES OF Cu and Zn IN AQUEOUS SOLUTION: THE INTERACTIVE EFFECTS OF MINE WASTEWATERMIXED MINERAL SYSTEMS EGIRANI, D.E 1, ANDREWS, J 2 AND JICKELLS,, T 2 1. FACULTY OF SCIENCE, NIGER DELTA UNIVERSITY WILBERFORCE ISLAND, NIGERIA 2. SCHOOL OF ENVIRONMENTAL SCIENCES, UNIVERSITY OF EAST ANGLIA, UK E-mail: eenonidavidson@yahoo.com IN PRESS IJRSR 209 OF 2013 HEALTH IMPLICATIONS Cu and Zn are important in human body metabolism, lead to poisoning at concentrations above safe limits. a deficiency can cause growth depression, in a community where Cu-Zn metal-mineral interaction exist, there is need to investigate the behavior of these metals and their complexes as best revealed by sorption LOCATION OF STUDY SITE CHARACTERISTICS OF MINE WATER FLOW DIAGRAM OF STUDIES ISOTHERM and % SORPTION where Kdtotal is the theoretical distribution coefficient for a 1:1 mixed suspension, (a)kaolinite/montmorillonite,(b) kaolinite/goethite and (c)montmorillonite/goethite Kdn is the distribution coefficient for n number of mineral suspensions, n is the number of mineral suspensions, ISOTHERM and % SORPTION % metal sorbed = where C1 and Ce are the initial and equilibrium metal concentrations ASSUMPTIONS differences between single and mixed mineral sorption are attributed to: development of secondary mineral phase during sorbate-sorbent interaction Activity of components minerals as individual networks or chemisorbed species. differential mass of mixed and single mineral phases QUANTITATIVE ANALYSIS Aqueous speciation calculations using phreeqc use a chemical composition for a solution as input and calculate the distribution of aqueous species and saturation indices for potential complexes. Cu(OH)2⇒ CuOH+ +OHZn(OH)2 ⇒ZnOH+ +OH- : ISOTHERM - PARYS MOUNTAIN MINE WATER actual and theoretical % sorption difference vs. pH of mixed mineral systems (a.kaolinite-mont, b. kaolgoethite and c. mont-goethite ISOTHERM- CWMRHEIDOL MINE WATER actual and theoretical % sorption difference vs. pH of mixed mineral systems (a.kaolinite-mont, b. kaolgoethite and c. mont-goethite Copper and Zinc hydroxylation Plot of saturation indices vs. pH of mine wastewater Conclusions None of the mineral systems reduced contaminants to U.S EPA levels, for humans and aquatic life, Parys, mineral mixing favors Cu sorption than Zn, Cwmrheidol, mixing favors Zn sorption than Cu, Cu and Zn sorption was a function of the mineral systems, mine water composition and pH THE END Abstract-Presentation ID# 216877 Presented by Dr. Davidson E. Egirani, at the 2013 Conference of the International Medical Geology Association (25–29 August 2013), Arlington, Virginia, U.S.A