03_Introduction_to_the_Atmosphere
advertisement

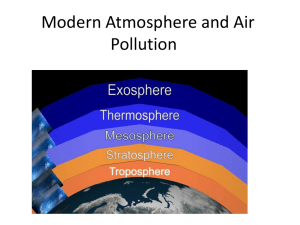
GEO 200: Physical Geography Introduction to the Atmosphere The Atmosphere • The Earth is unique because of the composition of its atmosphere, which makes life possible. – It supplies the oxygen that all but a handful of organisms need to survive. – It supplies the carbon dioxide that photosynthetic plants and animals use to make the carbon-based compounds required for living things. – It maintains the water supply for life. – It moderates the climate against temperature extremes. – It protects Earth from the Sun’s ultraviolet radiation. Air • Air is synonymous with atmosphere, and is a mixture of gases, mainly nitrogen and oxygen. – Also contains varying quantities of liquid and solid particles, such as soot. • Air is typically invisible: colorless, odorless, and tasteless. – Gaseous impurities can be smelled. – Suspended materials can be seen if in great enough concentration, such as water vapor in clouds. Vast ocean of air • The atmosphere completely surrounds Earth, like an ocean with land and water at its bottom. • It is held close to the surface by gravity, but because the strength of the attraction is low, the atmosphere is capable of vast, rapid motions. • The atmosphere interacts with other components of the Earth’s environment. Extent of atmosphere, part 1 • The atmosphere extends outward to 10,000 kilometers. • Most of its mass is concentrated at low elevations. – More than half of the atmosphere’s mass is below the elevation of the peak of Mount McKinley (6.2 kilometers). – More than 98 percent of the atmosphere’s mass lies within 26 kilometers of sea level. • Atmosphere fills empty spaces in rocks and soil, such as caves and crevices. Extent of atmosphere, part 2 • Gases are dissolved in the waters of the Earth as well as in the bodily and cellular fluids of living organisms. Gases of the Atmosphere • Basic composition of the atmosphere: – – – – Nitrogen – 78% Oxygen – 21% Argon – nearly 1% Neon, helium, methane, krypton, hydrogen, water vapor, carbon dioxide, ozone, carbon monoxide, sulfur dioxide, nitrogen oxides, and various hydrocarbons – trace amounts H20, CO2, and climate, part 1 • Only water (H20) vapor and carbon dioxide (CO2) have a significant effect on weather and climate. – Water vapor determines the humidity of the atmosphere, is the source of all clouds and precipitation, and is intimately involved in the storage, movement, and release of heat energy. – Water vapor and atmospheric CO2 significantly affect the climate because they can absorb infrared radiation, keeping the lower atmosphere warm. • The proportion of CO2 has been increasing at about 2 parts per million (ppm) per year and is at present about 370 ppm. H20, CO2, and climate, part 2 • It is debatable what exact effects this increase in atmospheric CO2 is having/will have on atmosphere, but most scientists believe it will make the lower atmosphere warm up enough to cause major global climatic changes. Ozone and other gases • Ozone is a molecule made up of three oxygen atoms (O3). It is concentrated in the ozone layer, where it helps to absorb deadly ultraviolet solar radiation and protect animal life. • The proportion of carbon monoxide, sulfur dioxide, nitrogen oxides, and various hydrocarbons is also increasing because of emissions from factories and cars. – All are hazardous to life and may have an effect on climate. Particulates, part 1 • Particulates are solid and liquid particles found in the atmosphere • They can be visible and/or invisible. • They come from both natural and human-made sources. – Larger particles are mainly water and ice. Particulates, part 2 • Particulates affect the weather and climate in two ways: – Many are hygroscopic (they absorb water), and water vapor collects around them, which contributes to cloud formation; – Particulates can either absorb or reflect sunlight, thus decreasing the amount of solar energy that reaches Earth’s surface. Vertical structure • Trailing bits first: – “–sphere” is used when talking about the entire layer; – When referring to just the upper portion of a layer or the boundary between two layers, “-pause” is used. • One way to define layers in the atmosphere is on the bases of temperature trends. There are five thermal layers in the atmosphere: troposphere. stratosphere, mesosphere, thermosphere, and exosphere. Thermal layers, part 1 • Temperature alternately decreases and increases from one layer to the next. • The thermal layers: – The troposphere is the lowest thermal layer of the atmosphere, in which temperature decreases with height. Most weather phenomena occur in the troposphere. • Height of the troposphere varies with time and place. – The tropopause is a transition zone at the top of the troposphere, where temperature ceases to decrease with height. Thermal layers, part 2 • The thermal layers (continued): – The stratosphere is the atmospheric layer directly above troposphere, where temperature increases with height. – The stratopause is the top of the stratosphere, elevation about 48 kilometers, were maximum temperature is reached. – The mesosphere is the atmospheric layer above the stratopause, where temperature again decreases with height as it did in the troposphere (note, this term also refers to the rigid part of the deep mantle, below the asthenosphere). Thermal layers, part 3 • The thermal layers (continued): – The mesopause is transition zone at the top of the mesosphere. – The thermosphere is the highest recognized thermal layer in the atmosphere, above the mesopause, where temperature remains relatively uniform for several kilometers and then increases continually with height. – The exosphere is the highest zone of Earth’s atmosphere. Thermal layers, part 4 • The warm zones of the thermal layers each have their own specific source of heat. – In the troposphere, it’s the visible portion of sunlight. – In the stratosphere and thermosphere, the Sun’s ultraviolet rays serve as the heat source (the warm zone of the stratosphere is near the top of the ozone layer, which absorbs UV rays). Atmospheric pressure • Atmospheric pressure is basically the weight of overlying air. Thus air pressure is normally highest at sea level and rapidly decreases with altitude. – One-half of all gas molecules making up the atmosphere are below 5.6 km, and 90 percent are below 16 km. – Above 80 km, atmospheric pressure is so slight that it cannot register on an ordinary barometer. Composition layers, part 1 • Principal gases of atmosphere have a uniform vertical distribution in the lowest 80 km. • The homosphere is the zone of homogeneous composition; in both troposphere and stratosphere. • The heterosphere is the zone of heterogeneous composition; begins in mesosphere and continues through exosphere; where gases tend to be layered according to their molecular masses rather than having the homogenous composition of the homosphere. Composition layers, part 2 • The ozonosphere is the ozone layer; the zone of relatively rich concentration of ozone in the atmosphere, between about 15 to 48 km high, that absorbs ultraviolet radiation. • The ionosphere is the deep layer of ions, electrically charged molecules and atoms, in mesosphere (middle and upper parts) and thermosphere (lower part) that aids in longdistance communication by reflecting radio waves back to Earth. Also generates auroral displays. Composition layers, part 2 • The ozonosphere is the ozone layer; the zone of relatively rich concentration of ozone in the atmosphere, between about 15 to 48 km high, that absorbs ultraviolet radiation. • The ionosphere is the deep layer of ions, electrically charged molecules and atoms, in mesosphere (middle and upper parts) and thermosphere (lower part) that aids in longdistance communication by reflecting radio waves back to Earth. Also generates auroral displays. Atmospheric change • Pollutants, inefficient and wasteful fossil fuel use, and rapid population growth are all contributing to changes in Earth’s atmosphere, as found by report of the World Conference on the Changing Atmosphere Implications for Global Security in 1988. • Report also predicted that we could expect severe economic and social dislocation because of these changes. Ozone depletion, part 1 • Ozone is naturally produced in the stratosphere and it serves to protect life on Earth by shielding it from the deadly ultraviolet rays of the Sun. – It is created in the upper atmosphere by the action of UV radiation. – UV-C radiation splits oxygen molecules into free oxygen atoms which then combine with oxygen molecules to form ozone. – In the stratosphere ozone molecules will be naturally broken down into oxygen molecules and free oxygen atoms by UV-B and UV-C radiation. Ozone depletion, part 2 • Ozone is naturally produced (continued). – The breakdown and formation of ozone is an ongoing process in this layer of the Earth’s atmosphere. • When produced in the troposphere, ozone harms life, where it damages tissues in humans (eyes, lungs, noses); it also damages vegetation and corrodes buildings. – Ozone is produced naturally in the stratosphere, while the combination of human activity such as automobile emissions and incoming solar radiation leads to its production in the troposphere. Ozone depletion, part 3 • When produced in the troposphere, ozone harms life (continued). – Depletion of stratospheric ozone increases the amount of tropospheric ozone. • Ozone in the stratosphere, lying in the ozone layer, is being depleted through a combination of natural and human-produced factors. – The natural include the oscillation of the stratospheric winds that occurs every 2.3 years, the 11-year sunspot cycle, volcanic debris from eruptions, and El Niño, which occurs every 3 to 5 years. Ozone depletion, part 4 • Ozone in the stratosphere . . . is being depleted (continued) – Chlorofluorocarbons (CFCs) are synthetic chemicals that affect ozone layer. • When ultraviolet radiation breaks down CFCs in the ozonosphere, the released chlorine then breaks down the ozone molecules, creating chlorine monoxide and oxygen molecules, which cannot filter solar radiation. Ozone Depletion Ozone depletion, part 5 • The ozone layer has been getting thinner since the 1960s, when CFCs came into major use as refrigeration and cooling agents. Then in 1979, a “hole” in ozone layer developed over Antarctica. Although it ebbs and flows each year, it has been increasing in size and duration each year. Then in 1980s, a similar “hole” developed over Arctic. Ozone depletion, part 6 • In 1978, many countries began banning use of CFCs, and by 1996, all countries of the industrial world had banned them. – Developing nations have an extended deadline of 2010 before they must ban them. • It is estimated that the current reservoir of CFCs in the atmosphere will persist for 50 to 100 years, continuing to deplete stratospheric ozone long after they are no longer produced or used. – Most research has predicted that the hole will not recover until sometime between 2040 and 2050. Weather vs. climate • Weather refers to short-run atmospheric conditions that exist for a given time in a specific area. • Climate refers to the pattern or aggregate of dayto-day weather conditions over a long period of days, encompassing both the average characteristics and the variations and extremes. Elements of weather/climate • There are four main elements, or variables, of weather and climate: – – – – Temperature Pressure Wind Moisture • All are measurable, vary frequently (if not continuously) in time and space, and provide the key to deciphering the complex mechanisms and process to weather and climate. Controls of weather/climate • There are seven principal controls that cause or influence the elements of weather and climate: – – – – – – – Latitude Distribution of land and water General circulation of the atmosphere General circulation of the oceans Elevations Topographic barriers Storms Latitude • Constantly changing positional relationship between Sun and Earth results in continuously changing amounts of solar energy that reach any given point on the surface of the Earth. • As a result, the basic distribution of heat energy over the surface of the Earth is a function of latitude. – Control latitude influences element temperature. Distribution of land and water • One of the most fundamental climatic differences is that between continental and maritime climates. – Oceans heat and cool more slowly and to a lesser extent than land masses, thus maritime climates experience milder temperatures than continental areas at similar latitudes. • Average January temperatures in Seattle, Wash., and Fargo, N.D., differ by as much as 18 degrees C., with Seattle’s average being 5 degrees C and Fargo’s being -13 degrees C. – Oceans also prolific source of atmospheric moisture, thus maritime climates more humid than continental climates. Circulation of the atmosphere • Atmosphere is in constant motion. – Flows range from transient breezes to regional wind patterns. – Semipermanent patterns of major wind and pressure patterns greatly influences most elements of weather and climate. • Simplifying the actual circulation shows that general surface wind direction varies according to latitude, with most surfaces winds in tropics coming from east, while those in middle latitudes flow mostly from the west. Circulation of the ocean • Movements of the oceans are analogous to motions of the atmosphere. • Motions range from minor ones to semipermanent currents affecting entire ocean basins. – For example, eastern coasts of continents have warmer currents while western coasts have cool currents. • Currents move warm water toward poles and cool water toward equator. • Ocean currents likewise affect climate. Elevation • Temperature, pressure, and moisture content generally decrease with elevation (altitude). • Decrease in these factors with elevation has significant effects on weather and climate, especially in mountain regions. Topographic barriers • Mountains and large hills can affect weather and climate by diverting wind flow and interrupting flow of moisture. • The windward side of a mountain range (the side facing the wind) often has a very different climate from the leeward side (the side sheltered from the wind). – This typically has a major effect on moisture and average rainfall, with one side being very wet and the other very dry. Storms • Storms result from interaction of the other climate controls, but then create their own specialized weather circumstances like other controls. – Some types of storms are predominant enough and frequent enough to affect climate as well as weather.