chapter_4_presentation
advertisement

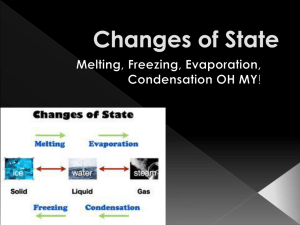
Viscosity What do melting chocolate, pancake syrup, ketchup, and ice water have in common? They are all liquids, and they all flow. The property that describes a liquid’s thinness or thickness is called viscosity. You can easily observe fluids such as water flowing out of a tap, milk being poured into a glass, or ketchup being squeezed out of a bottle. Your body contains many fluids, such as blood and the watery cytoplasm inside cells. It is more difficult to imagine gases flowing, but they do. Take a deep breath. What happened? Some of the air that surrounds you flowed into your lungs when your lungs and your ribcage expanded. Air flows out of your lungs when you exhale. Like liquids, gases flow and take up space. Therefore, gases and liquids can both be classified as fluids. The essentials of life (food, water, and air), are examples of substances that occur in the three different states of matter: solid, liquid, and gas. Solids have a definite shape and volume. Liquids have a definite volume, but no definite shape Gases have neither a definite volume nor a definite shape. The 5 major points of the particle theory are: 1. All matter is made up of very small particles. 2. All particles in a pure substance are the same. Different substances are made of different particles. 3. There is space between the particles. 4. The particles are always moving. 5. The particles in a substance are attracted to one another. The strength of the attractive force depends on the type of particle. A substance has a definite volume, but an indefinite shape. Is the substance a solid, liquid, or a gas? • According to the particle theory, we can think of solids as being made up of particles that are tightly packed together, like bees in a hive. • This way of thinking about the particles of a solid can explain why solids are greatly affected by gravity. • It explains why a solid will tumble toward the lowest surface when suspended in the air and then dropped. The particle theory suggests that the particles of a solid are so close together they cannot move around freely- they can only vibrate. Many solids can be ground into such small pieces that they can slip past each other when they are poured out of their containers. Sugar, salt, flour, dishwasher detergent, and Kool-Aid are examples of solids that can be poured. According to the particle theory, each tiny fragment of these solids contains billions of even smaller particles that are tightly packed together. Each tiny fragment is like a miniature solid in itself. This explains why solids form a pile when they are poured and why they do not keep flowing apart from each other. Again, according to particle theory, the particles that make up liquids have enough energy to pull away from each other and slide around each other, while at the same time vibrating close together in small clusters. Another way to think about what is happening on the level of the particles is to imagine groups of people at a party. The guests can move around by shifting as a group, or by flowing in between the other groups of partygoers. Similarly, liquid particles can slip past each other. Unlike the particles in solids, they do not form rigid structures. The particles of a liquid cannot hold their shape; instead, they fill a container and take the shape of that container. As in solids, liquid particles are so tightly packed together that they are easily affected by the downward pull of gravity. Therefore, liquid always flow to the lowest possible level. Liquids form a level surface when they are at rest. Some foods, like chocolate and ice cream, can be melted to form a liquid. Many other substances, like water, oil, syrup, and perfume, are liquids when they are at room temperature. All liquids can be transformed into their gaseous state when the liquids are heated. Many substances are gases when they are at room temperature; for example, the air around you is a gas. According to particle theory, gas particles are so far apart from each other that there is an enormous amount of empty space between them. Imagine sitting in a huge arena. One of your friends is also in the arena, but you are as far from one another as possible. This is what gas particles are like. In fact, the particle theory explains that most gases seem invisible to you because you are observing mostly empty space. This also explains why gas particles have no difficulty moving past one another, and why they flow very easily. The particle theory suggests that gas particles are so free to move that they do so in every direction, and they have a great deal of energy to move extremely far apart. Therefore, gas particles spread out so much that in a brief time, they fill up the space of an entire container or room. Since gases fill an entire room or container very quickly, they also take on the shape of the container in which they are sealed. However, gases do not flow to the lowest possible point like liquids do. Gas particles are not clustered or packed tightly together, so the energy of the particles allows them to move in all directions, sometimes even against gravity. For example, water vapour forms clouds that float in the sky. Unlike what happens to liquids, when the lid is taken off a container of gas, the gas particles will start to spread again, until they have filled the entire room or building. The particle theory can be used to explain how gases always occupy all the space that they can fillup, down, or sideways. How could you test whether or not a substance is a fluid? The process of melting is an example of a change of state, which occurs when the physical state of a substance is transformed into another state- for example, when a solid melts and becomes a liquid. The change from solid to liquid is called melting, and the change from liquid to gas is called vaporization. These changes occur when the substance is heated and the particles of the substance gain energy. If you were to cool the substance, the opposite would occur because the particles lose energy. The change from gas to liquid is called condensation, and the change from liquid to solid is called freezing. An unusual change of state occurs when a solid turns into its gaseous state without going through the liquid state. This change of state is known as sublimation. Dry ice is an example of sublimation- the particles of the ice gain energy and give off a fog. What is the difference between a gas and a vapour? A substance is called a gas if it exists as a gas at room temperature (for example, oxygen or carbon dioxide). The same substance is called a vapour if it normally exists as a solid or a liquid at room temperature (for example, water vapour). Evaporation is slow vaporization. It occurs over a wide range of temperatures. A wet towel will dry even if the air temperature is not high. On a cool day it will simply take longer for the water to evaporate from the towel. Boiling is rapid vaporization. It occurs at a specific temperature, called the boiling point. The boiling point of water is 100°C at sea level. Every substance has its own freezing point and melting point. The freezing point of water, for example, is 0°C at sea level. This is the temperature at which liquid water freezes. It is also the temperature at which ice melts. Classify the following items as either fluids (f), or non fluids (nf) Shampoo Balloon Thread Nail polish Perfume Blood Sap Smoke Sugar Air Ash Honey Natural gas Snow Some liquids can flow faster than others. Orange juice flows easily from a container, but honey is much slower. If you wanted to know how fast you could run, you would time yourself running a specific distance. In a similar way, you can measure how fast a fluid “runs”. You would measure the time it takes for the fluid to flow from one point to another. This characteristic is called the fluid’s flow rate. Why might it be important to know how to determine the flow rate, and therefore the viscosity of a liquid? The viscosity of liquids is an important property that must be measured precisely in some industries. For example, the viscosity of paints, varnishes and similar household products are closely regulated so that the paints and varnishes can be applied smoothly and evenly with a brush or a roller. The viscosity of some medicines, such as cough syrup, are regulated as well. Cough syrup has a high viscosity (but it’s still drinkable) so it will coat and soothe the throat. Your mouth is highly sensitive to viscosity, so food manufacturers ensure that ice cream toppings, pasta sauces, soups, gravies, salad dressings, and other products are just the right consistency (thickness) to suit consumers’ tastes. List two substances that have a low viscosity and two substances that have a high viscosity. What is the relationship between the viscosity of a liquid and its flow rate? Imagine that you and a group of friends are moving through a crowded shopping mall. Could you make your way through the crowd more quickly if your group were small rather than large? Small groups can move through a crowd more quickly than large groups because they can fit into the empty spaces between other groups more easily. In a similar way, particle theory suggests that small particles can move past each other more easily than large particles can. Large particles take up more space. Now imagine that you are back in the mall, but this time, everyone has shopping bags, bookbags, purses, and other parcels. It would be even more difficult to squeeze through the crowd because everyone would be taking up even more space. Similarly, some particles are bigger than others because of their shape. For example, oil particles are bulkier than water particles. For this reason, oil is more viscous than water. Now, imagine you’re back at the mall, but this time, everyone is wearing shoes made of Velcro. Every time you would try to walk by someone, your shoes would stick to theirs. With every step, you would have to stop and pull your foot away from another person’s foot. No matter how large or small the people in the crowd, it would take a long time to walk through the mall. Particle theory tells us that all particles attract each other, just like people wearing Velcro shoes. However, some particles attract and hold on to each other more tightly than other types. It is very difficult for these particles to flow past each other, so they do so very slowly. The strength of the attraction that particles have for each other is the most important factor in determining a fluid’s viscosity. Finally, imagine that everyone in the shopping mall is moving very, very slowly and won’t get out of the way when you and your friends try to go by. This is similar to what happens to particles when they are cooledthey slow down. Even though all fluids flow smoothly, they flow at different rates because they have different viscosities. Another way to define viscosity is “resistance to flow”. This means the particles can get around, but it may be difficult for them to pass one another; this resistance creates internal friction. A liquid’s ability to flow also depends on the energy that the particles have to move around. The warmer the liquid becomes, the more energy the particles have to move out of the way and make room for other particles to pass. However, as the temperature drops, the particles have less energy to move around, so the empty spaces between them get smaller and smaller. In general, a fluid’s viscosity decreases as the fluid is heated and increases as the fluid is cooled. Although gases, in general, flow much more easily than liquids, the viscosity of gases can vary too. The size and shape of gas particles and temperature are factors that affect the viscosity of gases as well as the viscosity of liquids. While an increase in temperature reduces viscosity in liquids, the opposite is true for gases. Gas particles do not depend on an increase in energy (a rise in temperature) to move father apart, as is the case for liquids. The particle theory suggests that gas particles are already very far apart. Extra energy increases the internal friction of gas particles because the particles speed up and collide with each other more frequently. Cooler temperatures in gases keep internal friction (and viscosity) low.