Climate change chemistry
advertisement

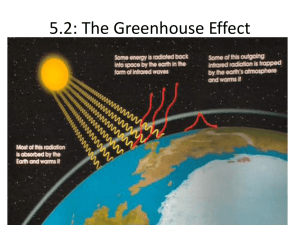
The Greenhouse Effect natural greenhouse effect http://www.elmhurst.edu/~chm/vchembook/globalwarmA5.html Greenhouse Analogy: Energy from the sun in the form of some ultraviolet and visible light (short wavelength) passes through the glass of the greenhouse. As the light strikes various surfaces in the greenhouse they are heated. These surfaces in turn re-radiate the heat in the form of infrared radiation (long wavelength). However, the IR radiation is blocked from escaping by the glass. IR is not able to pass through the glass, hence the greenhouse air heats up fairly dramatically. The greenhouse gases have the same property as the glass towards the IR radiation. Think of the greenhouse gases acting as an invisible glass shield around the earth. gases in the atmosphere Earth The sun emits ultraviolet (UV) radiation which hits the earth These rays of light hit the earth and reflect back towards space in the form of UV as well as Infrared (IR) radiation gases in the atmosphere pick up some of the heat from the IR rays and disperse them back into the earth’s atmosphere keeping the earth warm enough to sustain life Would you be able to feel the heat? On a molecular level… When certain gases in the atmosphere absorb IR Radiation their vibrational modes are excited and vibrate, causing them to collide with other molecules and transfer energy When the electrons return to their ground state, they re-emit the energy with a frequency equal to the frequency of energy gap between the two levels Only certain gases do this, just like dogs can hear frequencies that humans cannot because their eardrum vibrates with different wavelengths than ours If this didn’t happen… The climate would be an average of 60°F colder and the earth could not sustain life as we know it. So greenhouse gases are called greenhouse gases because they keep some heat in the atmosphere to sustain life on earth as a greenhouse does to sustain life in the greenhouse when it is cold outside Let’s find out which atmospheric gases are green house gases Greenhouse gas molecules are able to vibrate with the absorption of heat Natural Versus Enhanced Climate chemists believe that humans are producing more of the naturally occurring greenhouse gases than the atmosphere can naturally handle from… Anthropogenic The most abundant atmospheric gas, Nitrogen molecules have a strong bond which makes it chemically stable and non-reactive in most circumstances. Nitrogen's simple structure is unable to absorb either visible or infrared light. As a result, nitrogen is not a greenhouse gas. Oxygen is the second most abundant atmospheric gas. Why do you think that Oxygen is also not a greenhouse gas? greenhouse gases definition Greenhouse gases are gases that: • allow visible light and UV radiation (shortwavelength/high frequency) to pass through them • but (because of the nature of their covalent bonds in their molecules) absorb the infrared radiation (longer-wavelength radiation) of the same frequency as the one the Earth converts the energy from the Sun into and • reradiates this infrared radiation back to the Earth. Greenhouse factor • Compares ability of a greenhouse gas to absorb IR to the same amount of carbon dioxide which has a factor of 1. • Example: greenhouse factor of methane is 30 which means it absorbs as much as 30 molecules of carbon dioxide or 1 molecule of methane absorbs 30 times more as 1 molecule of carbon dioxide. greenhouse gas: CO2 • Sources: – Human: burning fossil fuels and wood, forest fires, burning waste – Natural: respiration, decay of organic matter, natural forest fires • Relative effect: Most important greenhouse gas (50% contribution) because of its great abundance (second largest after water vapour) and the large range of wavelengths over which it absorbs IR. Greenhouse gases: H2O • Sources: –Human: combustion of hydrocarbons –Natural: evaporation • Relative effect: 0.1 - Least effective in trapping radiation but is most abundant. Greenhouse gases: CH4 • Sources: – Human: cattle farming, rice paddies (wet soil means any organic matter in it is decomposed without oxygen) , petroleum and natural gas production. – Natural: digestive tracts of ruminants, cattle, bogs or marshes, bacterial fermentation – when organic matter is decomposed anaerobically, methane gas is produced. • Relative effect: 30 - Low abundance in atmosphere but it is more effective in absorbing infrared radiation, however, its atmospheric lifespan in the atmosphere is short. Greenhouse gases: N2O • Sources: – Human: use of nitrogen based fertilizers – Natural: bacterial action • Relative effect: 150 - Very effective in absorbing radiation, fairly long atmospheric life. Greenhouse gases: CFCs • Sources: – Human: refrigerators, air- conditioning, aerosols in spraying cans, foaming agents – Natural: none!!!! • Relative effect: 10 000 – 25 000 - Very effective in absorbing radiation, long atmospheric life but low abundance. Greenhouse gases: SF6 • Human source: electrical insulators • Greenhouse effect: 24 000 Some greenhouse gases are not naturally occurring – they are manmade Sulfur Hexafluoride Carbon tetrafluoride Hexafluoroethane CFC’s or Chlorofluorocarbons Effects of particulates • particulates scatters and reflect the incoming sunlight (visible and UV) so that less solar radiation enters the atmosphere; • particulates also cause a lowering of the temperature as they provide condensation nuclei around which water particles condense to form clouds reducing solar heating; • volcanic eruptions and large forest fires greatly increase the amount of particulates. So why do greenhouse gases have such a bad reputation? terminology • greenhouse effect = the absorbing of some of the infra-red radiation radiated from the Earth in the atmosphere which is then reradiated back to Earth; this results in… • global warming = a gradual increase in planet-wide temperatures Global warming effects (1) • World wide rise in sea levels resulting from: – Partial melting of glaciers and polar ice caps – Thermal expansion of water (as a result of heating). • Changes in crop yields: some crops will grow better, other worse. Global warming effects (2) • Changes in distribution of commercial crops • Changes in the distribution of pests and diseasecarrying organisms e.g. malaria. • More severe weather conditions: – floods in particular of coastal areas; more severe storms e.g. monsoon floods in Pakistan 2010 – More severe droughts e.g. 2010 worst drought in Amazon Global warming: evidence global temperatures since 1850s http://www.ncdc.noaa.gov/oa/climate/research/anomalies/anomalies.html • What can the mean global temperature anomaly be used for? This product is a global-scale climate diagnostic tool and provides a big picture overview of average global temperatures compared to a reference value. UV light in the atmosphere would break thte bonds of the Chlorine in the CFC’s and release it. Chlorine was found to deplete the ozone. HFC – Hydrofluorocarbons was the answer And Hydrofluorocarbons Which once saved the earth! Right now HFC’s do not contribute to climate change as much as CO2 but… Chemists to the rescue! Why should I care??????