Sulfur isotopes in biogechemical cycling
advertisement
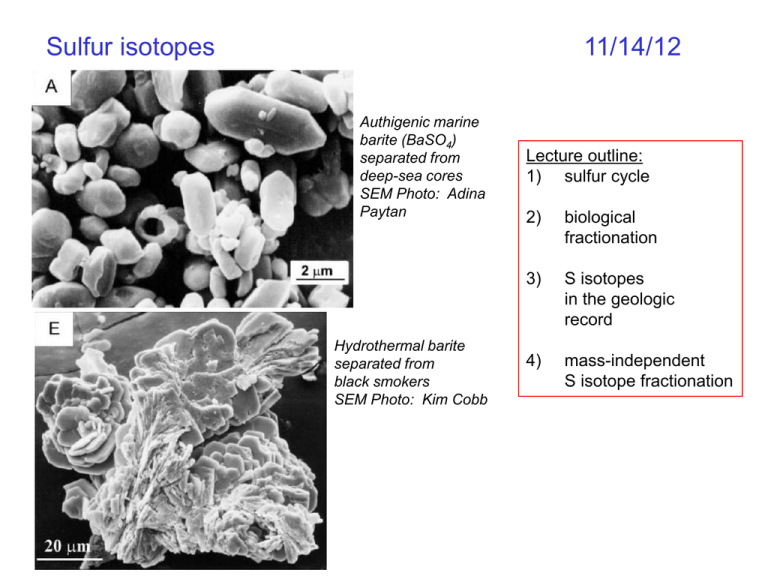
Sulfur isotopes 11/14/12 Authigenic marine barite (BaSO4) separated from deep-sea cores SEM Photo: Adina Paytan Hydrothermal barite separated from black smokers SEM Photo: Kim Cobb Lecture outline: 1) sulfur cycle 2) biological fractionation 3) S isotopes in the geologic record 4) mass-independent S isotope fractionation The sulfur cycle SO 2 From Don Wuebbles, Univ. Illinois UC, http://www.atmos.illinois.edu/courses/atms449-sp05/ Introduction to sulfur isotopes Sulfur stable isotopes: 32S: 95.02% 33S: 0.75% 34S: 4.21% 36S: 0.02% Sulfur isotope standard: Canyon Diablo Triolite 32S=0.9503957 33S=0.0074865 34S=0.0419719 36S=0.0001459 Five oxidation states: +6: e.g. BaSO4 +4: SO2 0: S (s) -1: FeS2 -2: e.g. H2S Rt marine sulfur = 20Ma Equilibrium fractionations relative to H2S Biologically-mediated SO4 reduction S6+ -naturally-occurring sulfides commonly depleted by 45 to 70‰! -bacterial sulfate reduction takes place in anoxic environments, where SO4 is reduced in place of O2 Thermochemical sulfate reduction - occurs at temps >100ºC -usually goes to near-completion -little fractionation 1000lnaH2S NOTE: the bacterial reduction of sulfate occurs via kinetic fractionation larger a S4+ S-1 Raleigh fractionation during sulfate reduction d34S of sulfate becomes heavier as light sulfide forms d34S of sulfide becomes heavier as sulfate source becomes heavier a HSOS 1.025 4 2 but a varies widely, depends on environmental conditions Use equations from Raleigh d18O lecture to calculate d34S of sulfate, sulfide as a function of fraction remaining. What would be the d34S of the total S at the end of the distillation? SO42- H2S(g) Equilibrium fractionations Bacterial Sulfate Reduction -15 to -70‰ depletion Thermochemical Sulfate Reduction -20‰ (at 100ºC) -15‰ (at 150ºC) -10‰ (at 200ºC) But you must know the starting d34S of the sulfate… AND… we can use mineral pairs to establish T of mineral formation ex: pyrite and chalcopyrite coprecipitated from same fluid but you must know the starting d34S of the sulfide…. BUT… the d34S of sulfide and sulfate in a solution depends on the relative proportions of H2S, HS-, and S2-, which depends on pH, O2 fugacity, total [S] SO… understanding present-day sulfur isotope variability in a given system is complicated …. Phanerozoic d34S evolution Cenozoic d34S evolution d34S and d13C not anti-correlated, as observed for last 1 billion years Paytan et al., 1998 Why anti-correlated over last 1Ga? increase burial C(org), = higher d13C =higher atmos. O2 =oxidize sulfides (low d34S) to SO4 =lower oceanic d34S atmospheric O2 did not change very much during the last 100Ma, so reduced S and C are not the only controls on atmospheric O2 Main factors that influence evolution of Cenozoic d34S: 1. deposition/burial of pyrite 2. deposition/burial of sulfates 3. intensity of hydrothermal activity and volcanism What happened at 55Ma? Why might this affect marine d34S? What does it mean that variations occur on timescales shorter than 20Ma (Rt of oceanic sulfur)? measured d34S of marine barite (BaSO4) Archean Sulfur isotopes and the hunt for early life Idea: If sulfur-reducing bacteria were around billions of years ago on Earth or Mars, shouldn’t large d34S excursions in sediments be measureable? Fact: Early work on Martian meteorites and Archean sediments revealed significant d34S excursions Mass-independent sulfur isotope fractionation Laboratory SO2 photolysis from Farquhar and Wing, 2003 Three-Isotope Plot MIF For mass-dependent fractionation (MDF): δ33S = 0.515×δ34S δ36S = 1.90×δ34S A new notation for deviation from the MDF line: 33S = δ33S− 0.515×δ34S 36S = δ36S− 1.90×δ34S MDF 33S Evolution of the atmosphere: multiple isotopes and MIFs Ono, 2008 keep in mind uncertainties… Johnston, 2011 Archean mass-independent sulfur isotope fractionation 33S = departure from mass fractionation line (MFL) = 0 present-day but highly variable in Archean sediments Today atmospheric mass-independent rxns occur, but isotopes are re-mixed in surface and biological redox chemistry, so 33S = 0 in all sediments Models suggest that atmospheric O2 had to be less than 10-5 Pa at 3Ga <1% of present-day Farquhar & Thiemens, 2000,2001 Archean mass-independent sulfur isotope fractionation the “Great Oxygenation Event (GOE)” from Lyons & Reinhard, 2011 Early Earth sulfur cycle: uncertainties abound! from Farquhar and Wing, 2003 Snowball Earth and the Sulfur Cycle planet cools considerably, incipient glaciation, ice grows near 30 runaway ice albedo makes snowball rising CO2 increases temp., melts ice, reverse ice albedo feedback temporary hothouse Earth after snowball Cap carbonate overlying diamictite; photo by Francis MacDonald translates into progressive enrichment of oceans by continued burial of pyrite in ocean from Hurtgen et al., 2002 anomaly upon deglaciation should be recorded in cap carbonates from Hurtgen et al., 2002 cap carbonates from Hurtgen et al., 2002