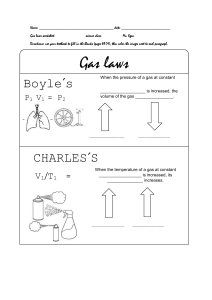
Name: period: Charles’s Law practice problems Charles’ Law Charles’ Law states that if a gas is heated up and the pressure does not change, the volume will. So, for a fixed mass of gas at a constant pressure, volume ÷ temperature will remain the same. Boyle’s Law Boyle’s Law states that for a gas at a constant temperature, pressure × volume is also constant. So, increasing pressure means that volume will decrease providing that the temperature remains constant. ……………………………………………………………………………………………………………………………………….. where: V1=is the starting volume (measured in any relevant unit of volume T1= is the starting temperature (must be in Kelvin) V2= is the finishing pressure (same units as T2= is the finishing temperature (must be in Kelvin) Name: period: Charles’s Law practice problems Practice questions 1. A container holds 50.0 mL of nitrogen at 25oC and a pressure of 736 mmHg. What will the volume be if the temperature increases by 35oC? 2. A sample of oxygen occupies a volume of 160 L at 91oC. What will be the volume of oxygen when the temperature drops to 0.00oC? 3. A sample of hydrogen has an initial temperature of 50oC. When the temperature is lowered to 5.0oC, the volume of the hydrogen becomes 212 cm3. What is the initial volume of the hydrogen? 4. 568 cm3 of chlorine at 25oC will occupy what volume at -25oC while the pressure remains constant. 5. A sample of helium has a volume of 521 L at a pressure of 75 kPa and a temperature of 18oC. When the temperature is increased to 23oC what is the volume of the helium?