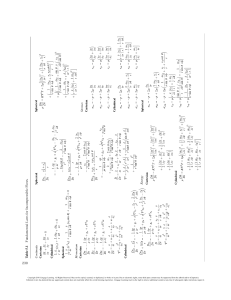
Fundamentals of Analytical Chemistry, 10e Chapter 23: Instruments for Optical Spectrometry [Author Name], [Book Title], [#] Edition. © [Insert Year] Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May a publicly accessible website, in whole or in part. not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 1 Chapter Objectives (1 of 3) By the end of this chapter, you should know: • Sources for spectrochemical measurements. • Types of optical materials. • How to use laser sources for spectroscopy. • How to use monochromators for wavelength selection. • The role of interference and interference filters. • How to use diffraction gratings. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 2 Chapter Objectives (2 of 3) • How to use detectors of radiant energy. • Types of transducers and detectors and their uses. • The role of the signal-to-noise ratio of spectrometric measurements. • How to use phototubes and photomultiplier tubes. • How to use silicon photodiodes. • How to use charge-coupled and charge-injection devices. • How to use thermal detectors. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 3 Chapter Objectives (3 of 3) • How to use sample containers for UV/visible measurements. • How to use single- and double-beam spectrophotometers. • How to use infrared spectrophotometers. • How to use interferometers for infrared spectrophotometry. • How to use Fourier transform infrared spectroscopy. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 4 Important Equations For grating For a filter Transducer response nλ = d(sin i + sin r) 2t max n G = KP + K′ Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 5 Introduction • Because the basic components of analytical instruments for absorption as well as for emission and fluorescence spectroscopy are similar in function and in general performance requirements regardless of whether they are designed for UV, visible, or IR radiation, they are all frequently called optical instruments. • Spectroscopy in the UV/visible and IR regions is often called optical spectroscopy. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 6 23A Instrument Components (1 of 6) • Most spectroscopic instruments in the UV/visible and IR regions have five components: 1. a stable source of radiant energy 2. a wavelength selector 3. one or more sample containers 4. a radiation detector to convert radiant energy to a measurable electrical signal 5. a signal-processing and readout unit Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 7 23A Instrument Components (2 of 6) • Figure 23-1 illustrates how these components are configured to make optical spectroscopic measurements. • Figure 23-1a shows the arrangement for absorption measurements. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 8 23A Instrument Components (3 of 6) • Figure 23-1b illustrates the configuration for fluorescence measurements. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 9 23A Instrument Components (4 of 6) • Figure 23-1c illustrates the configuration for emission spectroscopy. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 10 23A Instrument Components (5 of 6) • An external source of radiation is required for absorption and fluorescence spectroscopy (Figures 23-1a and b). • In absorption measurements, the attenuation of the source radiation at the selected wavelength is measured. • In fluorescence measurements, the source excites the analyte and causes the emission of characteristic radiation that is usually measured perpendicular to the incident source beam. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 11 23A Instrument Components (6 of 6) • In emission spectroscopy (Figure 23-1c), the sample itself is the emitter. No external radiation source is needed. The sample is usually introduced into a plasma or flame that provides enough thermal energy to cause the analyte to emit characteristic radiation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 12 23A-1 Optical Materials • The cells, windows, lenses, mirrors, and wavelength-selecting elements in an optical spectroscopic instrument must transmit or reflect radiation in the wavelength region being investigated. • Figure 23-2 shows the functional wavelength ranges for several optical materials that are used in the UV, visible, and IR regions of the spectrum. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 13 23A-2 Spectroscopic Sources (1 of 6) • For a source to be suitable for spectroscopic studies, it must generate a beam of radiation sufficiently powerful for detection and measurement. its output power should be stable for periods of time, which generally requires a well-regulated power supply. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 14 23A-2 Spectroscopic Sources (2 of 6) • • There are two major types of sources, as illustrated in Figure 23-3. 1. Continuum sources emit radiation that changes in intensity only slowly as a function of wavelength. 2. Line sources, which emit a limited number of spectral lines that each span only a very narrow range. A continuum source provides a broad distribution of wavelengths within a particular spectral range known as a spectral continuum. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 15 23A-2 Spectroscopic Sources (3 of 6) • Table 23-1 shows examples of continuum sources suitable for various types of spectroscopy. Table 23-1 Continuum Sources for Optical Spectroscopy Source Wavelength Region, nm Type of Spectroscopy Xenon arc lamp 250−600 Molecular fluorescence H2 and D2 lamps 160−380 UV molecular absorption Tungsten/halogen lamp 240−2500 UV/visible/near-IR molecular absorption Tungsten lamp 350−2200 Visible/near-IR molecular absorption Nernst glower 400−20,000 IR molecular absorption Nichrome wire 750−20,000 IR molecular absorption Globar 1200−40,000 IR molecular absorption Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 16 23A-2 Spectroscopic Sources (4 of 6) • Sources can also be classified as: Continuous sources that emit radiation continuously with time. Pulsed sources that emit radiation in bursts. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 17 23A-2 Spectroscopic Sources (5 of 6) Two examples of continuum sources with their spectra are tungsten filament lamps (Figure 23-4; left) and deuterium lamps (Figure 23-5; right). Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 18 Feature 23-1: Laser Sources: The Light Fantastic (1 of 8) Lasers have become widely used as sources in certain types of analytical spectroscopy. To help understand how a laser works, consider an assembly of atoms or molecules interacting with an electromagnetic wave. For simplicity, consider the atoms or molecules to have two energy levels: an upper level 2 with energy E 2 and a lower level 1 with energy E1. If the electromagnetic wave is of a frequency corresponding to the energy difference between the two levels, excited species in level 2 can be stimulated to emit radiation of the same frequency and phase as the original electromagnetic wave. Each stimulated emission generates a photon while each absorption removes a photon. The number of photons per second, called the radiant flux φ, changes with distance as the radiation interacts with the assembly of atoms or molecules. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 19 Feature 23-1: Laser Sources (2 of 8) The change in flux, dφ, is proportional to the flux itself; to the difference in the populations of the levels, n2 n1; and to the path length of the interaction, dz, according to where k is a proportionality constant related to the absorptivity of the absorbing species. d k n2 n1 dz If the upper-level population can be made to exceed that of the lower level, there will be a net gain in flux, and the system will behave as an amplifier. If n2 n1, the atomic or molecular system is said to be an active medium and to have undergone population inversion. The resulting amplifier is called a laser, which stands for light amplification by stimulated emission of radiation. The optical amplifier can be converted into an oscillator by placing the active medium inside a resonant cavity made from two mirrors as shown in Figure 23F-1. When the gain of the active medium equals the losses in the system, laser oscillation begins. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 20 Feature 23-1: Laser Sources (3 of 8) Figure 23F-1 Laser cavity. The electromagnetic wave travels back and forth between the mirrors, and the wave is amplified with each pass. The output mirror is partially transparent to allow only a fraction of the beam to pass out of the cavity. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 21 Feature 23-1: Laser Sources (4 of 8) Population inversion is often achieved by a multilevel atomic or molecular system in which the excitation process, called pumping, is accomplished by electrical means, by optical methods, or by chemical reactions. In some cases, the population inversion can be sustained to produce a continuous wave (CW) output beam that is continuous with respect to time. In other cases, the lasing action is self-terminating so that the laser is operated in a pulsed mode to produce a repetitive pulse train or a single shot action. There are many types of lasers available. The first operating lasers were solid-state lasers in which the active medium was a ruby crystal. In addition to the ruby laser, there are many other solid-state lasers. A widely used material contains a small concentration of Nd3 embedded in a yttrium-aluminum-garnet (YAG) host. The active material is shaped into a rod and pumped optically by a flashlamp, as illustrated in Figure 23F-2a. The pump and laser transitions are shown in Figure 23F-2b. The Nd:YAG laser generates nanosecond pulses with a very high output power at a wavelength of 1.06 μm. The Nd:YAG laser is popular as a pumping source for tunable dye lasers. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 22 Feature 23-1: Laser Sources (5 of 8) Figure 23F-2 Schematic of a Nd:YAG laser (a) and energy levels (b). The pump transitions are in the red region of the spectrum, and the laser output is in the near infrared. The laser is flashlamp pumped. The region between the two mirrors is the laser cavity. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 23 Feature 23-1: Laser Sources (6 of 8) Several other rare earth elements, such as ytterbium, holmium, and erbium, are also used as dopants in solid-state lasers. Titanium-doped sapphire (Ti:sapphire) is used to produce a tunable infrared laser. Some versions generate ultrashort pulses of very high output power. The very common helium-neon (He-Ne) laser is a gas laser that operates in a CW mode. The He-Ne laser is widely used as an optical alignment aid and as a source for some types of spectroscopy. The nitrogen laser lases on a transition of the nitrogen molecule at 337.1 nm. It is a self-terminating pulsed laser that requires a very short electrical pulse for pumping the appropriate transitions. The N2 laser is also used for pumping tunable dye lasers, as discussed later. Excimer (excited dimer or trimer) lasers are among the newest gas lasers. Rare-gas halide excimer lasers were first demonstrated in 1975. In one popular type, a gas mixture of Ar, F2 , and He produces ArF excimers when subjected to an electrical discharge. The excimer laser is an important UV source for photochemical studies, for fluorescence applications, and for pumping tunable dye lasers. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 24 Feature 23-1: Laser Sources (7 of 8) Dye lasers are liquid lasers containing a fluorescent dye such as one of the rhodamines, a coumarin, or a fluorescein. These have been made to lase at wavelengths from the IR to the UV. Lasing typically occurs between the first excited singlet state and the ground state. The lasers can be pumped by flashlamps or by another laser such as those discussed previously. Lasing can be sustained over a continuous range of wavelengths on the order of 40 to 50 nm. The broad band over which lasing occurs makes the dye laser suitable for tuning by inserting a grating, a filter, a prism, or an interferometric element into the laser cavity. Dye lasers are very useful for molecular fluorescence spectroscopy and many other applications. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 25 Feature 23-1: Laser Sources (8 of 8) Semiconductor lasers, also known as diode lasers, obtain population inversion between the conduction band and the valence band of a pn-junction diode. Various compositions of the semiconductor material can be used to give different output wavelengths. Diode lasers can be tuned over small wavelength intervals and can produce outputs in the IR region of the spectrum. They have become extremely useful in CD and DVD players, in CD-ROM drives, in laser printers, and in spectroscopic applications, such as Raman spectroscopy. Laser radiation is highly directional, spectrally pure, coherent, and highly intense. These properties have made possible many unique research applications that cannot easily be achieved with conventional sources. Despite the many advances in laser science and technology, only recently have lasers become routinely useful in analytical instruments. Even today, many high-powered or ultrafast lasers can be somewhat difficult to align, maintain, and use. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 26 23A-2 Spectroscopic Sources (6 of 6) • The continuum sources for IR radiation are normally heated insert solids. • A Globar source consists of a silicon carbide rod that emits IR radiation when heated to about 1500○C by passing electricity through it. • A Nernst glower is a cylinder of zirconium and yttrium oxides that emits IR radiation when heated to a high temperature by an electric current. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 27 23A-3 Wavelength Selectors (1 of 17) • Spectroscopic instruments in the UV and visible regions are usually equipped with one or more devices to restrict the radiation being measured to a narrow band that is being absorbed or emitted by the analyte. These devices enhance selectivity and sensitivity of the instrument. For absorption measurements, narrow bands of radiation diminish the change for Beer’s law deviations due to polychromatic radiation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 28 23A-3 Wavelength Selectors (2 of 17) • To restrict the radiation being measured, instruments can use A monochromator or a filter to isolate the desired wavelength band. A spectrograph to spread out the wavelengths so they can be detected with a multichannel detector. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 29 23A-3 Wavelength Selectors (3 of 17) • A monochromator uses a grating to disperse a spectrum. It contains an entrance slit and an exit slit. The exit slit is used to isolate a small band of wavelengths. One band at a time is isolated and different bands can be transmitted sequentially by rotating the grating. The wavelength ranged passed by this device is the spectral bandpass or effective bandwidth. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 30 23A-3 Wavelength Selectors (4 of 17) • Figure 23-6 shows two types of monochromators: (a) grating monochromator. (b) prism monochromator. • Monochromators generally have diffraction grating to disperse radiation into component wavelengths. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 31 23A-3 Wavelength Selectors (5 of 17) • Figure 23-6a shows the path of radiation through a typical grating monochromator. • Angular dispersion results from diffraction at the reflective surface of the refraction grating. • The two wavelengths are focused by the second concave mirror onto the focal plane, where they appear as two images on the entrance slit. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 32 23A-3 Wavelength Selectors (6 of 17) • A spectrograph uses a grating to disperse a spectrum. It contains an entrance slit to define the area of the source to be viewed. A large opening at its exit allows a range of wavelengths to strike a multiwavelength detector. • When an instrument contains a spectrograph, the sample and wavelength are reversed from the configuration shown in Figure 23-1a. • A spectrograph contains a diffraction grating but has no exit slit, so the dispersed spectrum impinges on a multiwavelength detector. • A polychromator contains multiple exit slits so that several wavelength bands can be isolated simultaneously. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 33 23A-3 Wavelength Selectors (7 of 17) • The effective bandwidth of a wavelength selector is the width of the band of radiation in wavelength units at half-peak height. • Figure 23-7 shows effective bandwidth in the exit slit output as a monochromator is scanned from λ1 Δλ to λ1 Δλ. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 34 23A-3 Wavelength Selectors (8 of 17) • The echellette grating is one of the most common types of reflection gratings. • Figure 23-8 shows a magnified cross-sectional view of a few typical grooves in an echellette-type grating. • The grating is grooved or blazed so that it has relatively broad faces where reflection occurs and narrow unused faces to provide highly efficient diffraction of radiation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 35 23A-3 Wavelength Selectors (9 of 17) • In Figure 23-8, a parallel beam of monochromatic radiation approaches the grating surface at an angle i relative to the grating normal. The incident beam is made up of three parallel beams that make up a wave front labeled 1, 2, and 3. • The diffracted beam is reflected at the angle r, which depends on the wavelength of radiation. • Equation 23-1 shows the relationship between the angle of reflection r and the wavelength of the incoming radiation. nλ = d(sin i + sin r) (23-1) Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 36 23A-3 Wavelength Selectors (10 of 17) • The echellette grating is blazed for use in relatively low orders, but an echelle grating is used in high orders (>10). • The echelle grating is often used with a second dispersive element, such as a prism, to sort out overlapping orders and provide cross dispersion. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 37 23A-3 Wavelength Selectors (11 of 17) • As shown in Figure 23-9, a major advantage of a grating monochromator over a prism monochromator is that the dispersion along the focal plane is, for all practical purposes, linear. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 38 23A-3 Wavelength Selectors (12 of 17) • Reciprocal linear dispersion is the change in wavelength per unit distance along the focal point of the monochromator. • The product of reciprocal linear dispersion and slit width is the spectral or effective bandpass of the monochromator. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 39 23A-3 Wavelength Selectors (13 of 17) • Gratings can be formed on a concave surface in much the same way as on a plane surface. • A concave grating permits the design of a monochromator without auxiliary collimating and focusing mirrors or lenses because the concave surface both disperses the radiation and focuses it on the exit slit. • Holographic gratings are produced using an optical technique and are not subject to mechanical errors found in other gratings. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 40 Example 23-1 (1 of 2) An echellette grating containing 1450 blazes per millimeter was irradiated with a polychromatic beam at an incident angle 48 degrees to the grating normal. Calculate the wavelengths of radiation that would appear at angles of reflection of +20, +10, and 0 deg (angle r, Figure 23-8). Solution To obtain d in Equation 23-1, write d 1 mm nm 10 6 1450 blazes mm 689.7 nm blaze When r in Figure 23-8 equals +20 deg, λ can be obtained by substituting into Equation 231. Therefore, 689.7 nm 748.4 nm sin 48 sin 20 n n and the wavelengths for the first-, second-, and third-order reflections are 748, 374, and 249 nm, respectively. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 41 Example 23-1 (2 of 2) Similar calculations, shown in the table that follows, reveal that the wavelength in the second order is one half that in the first order, the wavelength in the third order is one third that in the first order, and so forth. Wavelength (nm) for r, deg n=1 n=2 n=3 20 748 374 249 10 632 316 211 0 513 256 171 Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 42 23A-3 Wavelength Selectors (14 of 17) • Figure 23-10 shows two types of filters used in spectroscopy: 1. Interference filters. 2. Absorption filters. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 43 23A-3 Wavelength Selectors (15 of 17) • Interference filters rely on optical interference to provide a relatively narrow band of radiation. • Figure 23-11 shows the structure of an interference filter and an example of conductive interference. • The filter contains a thin layer of transparent dielectric material. • A dielectric is a nonconducting substance or insulator that is usually optically transparent. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 44 23A-3 Wavelength Selectors (16 of 17) • Equation 23-2 gives the nominal wavelength for an interference filter λmax 2t max n (23-2) where t is the thickness of the central dielectric layer, η is its refractive index, and n is an integer called the interference order. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 45 23A-3 Wavelength Selectors (17 of 17) • Absorption filters are limited to use in the visible region. • They usually consist of a colored glass plate that absorbs part of the incident radiation and transmits the desired band of wavelengths. • One filter can only isolate a single band of wavelengths, so a new filter must be used for a different wavelength band. • In the IR region of the spectrum, an interferometer is used. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 46 23A-4 Detecting and Measuring Radiant Energy (1 of 27) • To obtain spectroscopic information, radiant power transmitted, fluoresced, or emitted must be detected and converted into a measurable quantity. • A detector indicates, identifies, or records changes in a physical or chemical quantity in its environment. • A transducer converts various types of chemical and physical quantities into electrical signals. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 47 23A-4 Detecting and Measuring Radiant Energy (2 of 27) • The ideal transducer for electromagnetic radiation responds rapidly to low levels of radiant energy over a broad wavelength range. produces an electrical signal that is easily amplified with a low electrical noise level. produces an electrical signal linearly related to the radiant power P of the beam as shown in Equation 23-3. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 48 23A-4 Detecting and Measuring Radiant Energy (3 of 27) • The other variables in Equation 23-3 are G: the electrical response of the detector in units of current, voltage, or charge. K: a proportionality constant that measures sensitivity in terms of electrical response per unit of radiant power input. K′: dark current, which is a current produced by some radiation transducers when no light strikes the device. G KP K (23-3) Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 49 23A-4 Detecting and Measuring Radiant Energy (4 of 27) • Because instruments with a significant dark current response are usually equipped with an electronic circuit or computer program to automatically subtract the dark current, Equation 23-3 can usually be simplified to Equation 23-4. G = KP (23-4) Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 50 23A-4 Detecting and Measuring Radiant Energy (5 of 27) • Table 23-2 shows common detectors for absorption spectroscopy and divides transducers into two types: photo detectors and thermal detectors. Type Wavelength Range, nm Photon Detectors Phototubes 150–1000 Photomultiplier tubes 150–1000 Silicon photodiodes 350–1100 Photoconductive cells 1000–50,000 Thermal Detectors Thermocouples 600–20,000 Bolometers 600–20,000 Pneumatic cells 600–40,000 Pyroelectric devices 1000–20,000 Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 51 23A-4 Detecting and Measuring Radiant Energy (6 of 27) • Photon detectors are based on the interaction of radiation with a reactive surface to produce electrons (photoemission) or to promote electrons to energy states in which they can conduct electricity (photoconduction). • Only UV, visible, and near-IR radiation possess enough energy to cause photoemission to occur. • Photoconductors can be used in the near-, mid-, and far-IR region of the spectrum. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 52 23A-4 Detecting and Measuring Radiant Energy (7 of 27) • Two methods are commonly used to detect IR radiation: 1. measuring the temperature rise of a thermally sensitive material. 2. measuring the increase in electrical conductivity of a photoconducting material. • Because the temperature changes involved are tiny, ambient temperature must be carefully controlled to avoid large errors. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 53 Feature 23-5: Signals, Noise, and the Signalto-Noise Ratio (1 of 3) The output of an analytical instrument fluctuates in a random way. These fluctuations limit the precision of the instrument and are the net result of a large number of uncontrolled random variables in the instrument and in the chemical system under study. An example of such a variable is the random arrival of photons at the photocathode of a photomultiplier tube. The term noise is used to describe these fluctuations, and each uncontrolled variable is a noise source. The term comes from audio and electronic engineering where undesirable signal fluctuations appear to the ear as static, or noise. The average value of the output of an electronic device is called the signal, and the standard deviation of the signal is a measure of the noise. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 54 Feature 23-5: Signals, Noise, and the Signalto-Noise Ratio (2 of 3) An important figure of merit for analytical instruments, stereos, compact-disk players, and many other types of electronic devices is the signal-to-noise ratio (S/N ). The signalto-noise ratio is usually defined as the ratio of the average value of the output signal to its standard deviation. The signal-to-noise behavior of an absorption spectrophotometer is illustrated in the spectra of hemoglobin shown in Figure 23F-4. The spectrum at the bottom of the figure has S/N = 100, and you can easily pick out the absorption maxima at 540 nm and 580 nm. As the S/N degrades to about two in the second spectrum from the top of the figure, the peaks are barely visible. Somewhere between S/N = 2 and S/N = 1, the peaks disappear altogether into the noise and are impossible to identify. As modern instruments have become computerized and controlled by sophisticated electronic circuits, various methods have been developed to increase the signal-to-noise ratio of instrument outputs. These methods include analog filtering, lock-in amplification, boxcar averaging, smoothing, and Fourier transformation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 55 Feature 23-5: Signals, Noise, and the Signalto-Noise Ratio (3 of 3) Figure 23F-4 Absorption spectra of hemoglobin with identical signal levels but different amounts of noise. Note that the curves have been offset on the absorbance axis for clarity. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 56 23A-4 Detecting and Measuring Radiant Energy (8 of 27) • The response of a phototube or photomultiplier tube results from the photoelectric effect. • Figure 23-12 shows the structure of a phototube. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 57 23A-4 Detecting and Measuring Radiant Energy (9 of 27) • When a voltage is applied across the electrodes of a phototube, the emitted photoelectrons are attracted to the positively charged wire anode and produce a photocurrent. • Photoelectrons are electrons that are ejected from a photosensitive surface by electromagnetic radiation. A photocurrent is the current in an external circuit that is limited by the rate of ejection of photoelectrons. • The photocurrent produced can be amplified and measured. • The number of photoelectrons ejected from the photocathode per unit time is directly proportional to the radiant power of the beam striking the surface. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 58 23A-4 Detecting and Measuring Radiant Energy (10 of 27) • The photomultiplier tube (PMT) resembles the phototube but is more sensitive. • In place of a single wire anode, the PMT has a series of electrodes called dynodes as shown in Figure 23-13. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 59 23A-4 Detecting and Measuring Radiant Energy (11 of 27) • With modern electronic instruments, it is possible to detect the electron pulses resulting from the arrival of individual photons at the photocathode of a PMT. • The pulses are counted, and the accumulated count is a measure of the intensity of the electromagnetic radiation impinging on the PMT. • Photon counting is advantageous when the light intensity, or frequency of arrival of photons at the photocathode, is low. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 60 23A-4 Detecting and Measuring Radiant Energy (12 of 27) • Photoconductive transducers consist of a thin film of a semiconductor material, deposited often on a nonconducting glass surface and sealed in an evacuated envelope. A semiconductor is a substance having conductivity that lies between that of a metal and that of a dielectric (an insulator). A photoconductor is typically placed in series with a voltage source and load resistor. Voltage drop across the load resistor serves as a measure of the radiant power of the radiation beam. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 61 23A-4 Detecting and Measuring Radiant Energy (13 of 27) • Crystalline silicon is a semiconductor and a Group IV element. In a silicon crystal, each of the four valence electrons of silicon is combined with electrons from four other silicon atoms to form four covalent bonds. At room temperature, sufficient thermal agitation occurs to liberate an occasional electron from its bonded state so that it is free to move throughout the crystal. Thermal excitation of an electron leaves behind a positively charged hole that is also mobile. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 62 23A-4 Detecting and Measuring Radiant Energy (14 of 27) • Crystalline silicon is a semiconductor and a Group IV element. The mechanism of hole movement is stepwise, with a bound electron from a neighboring silicon atom jumping into the electron-deficient hole and creating another positive hole in its wake. The motion of electrons and holes in opposite directions in semiconductors is the source of conduction in these devices. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 63 23A-4 Detecting and Measuring Radiant Energy (15 of 27) • Doping enhances the conductivity of silicon. 1. A tiny controlled amount of a Group V or Group III element is distributed homogeneously throughout a silicon crystal. Valence electrons of the dopant form covalent bonds with the silicon atoms. 2. Group V elements have four valence electrons that bond with the four valence electrons of silicon, leaving one electron free to conduct. 3. Group III elements have three valence electrons available to bond with the valence electrons for silicon, producing an excess of holes. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 64 23A-4 Detecting and Measuring Radiant Energy (16 of 27) • An n-type semiconductor contains unbonded electrons (negative charges), as shown in Figure 23-14 (left). Electrons are the majority carrier. • A p-type semiconductor contains an excess of holes (positive charges), as shown in Figure 23-15 (right). Holes are the majority carrier. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 65 23A-4 Detecting and Measuring Radiant Energy (17 of 27) • It is possible to fabricate a pn junction or a pn diode, which is conductive in one direction but not the other. • Electrical wires are attached to each end of the device. • Figure 23-16a shows a schematic of a silicon diode. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 66 23A-4 Detecting and Measuring Radiant Energy (18 of 27) • Figure 23-16b shows the junction in its conduction mode, wherein the positive terminal of a dc source is connected to the p region and the negative terminal to the n region. • The diode is forward biased under these conditions. • Bias is a dc voltage, sometimes called a polarizing voltage, applied to a circuit element to establish a reference level for operation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 67 23A-4 Detecting and Measuring Radiant Energy (19 of 27) • In Figure 23-16b, the excess electrons in the n region and the positive holes in the p region move toward the junction, where they combine and annihilate each other. • The negative terminal of the source injects new electrons into the n region to continue the conduction process. • The positive terminal extracts electrons from the p region, creating new holes that can migrate toward the pn junction. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 68 23A-4 Detecting and Measuring Radiant Energy (20 of 27) • Photodiodes are semiconductor pn-junction devices that respond to incident light by forming electron-hole pairs. • When voltage is applied to the p diode such that the p-type semiconductor is negative with respect to the n-type semiconductor, the diode is said to be reverse biased. • The majority of electrons are drawn away from the junction, leaving a nonconductive depletion layer. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 69 23A-4 Detecting and Measuring Radiant Energy (21 of 27) • Figure 23-16c shows the behavior of a silicon junction that is reverse biased. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 70 23A-4 Detecting and Measuring Radiant Energy (22 of 27) • Diode-array detectors make high-speed spectroscopy possible. • One thousand or more silicon photodiodes can be fabricated side by side on a small silicon chip. Wavelengths can be monitored simultaneously using diode-array detectors placed along the length of the focal plane of a monochromator. • If the number of light-induced charges per unit time is large compared to thermally produced charge carriers, the current in an external circuit, under reverse-bias conditions, is directly related to the incident radiant power. • Added image intensifiers provide gain and allow detection of low light levels. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 71 23A-4 Detecting and Measuring Radiant Energy (23 of 27) • Figure 23-17 is a cross-sectional depiction of one of the pixels in a charge transfer device. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 72 23A-4 Detecting and Measuring Radiant Energy (24 of 27) • In a charge-injection device (CID) detector, the voltage change arising from movement of the charge from the region under one electrode to the region under the other is measured. • In a charge-coupled device (CCD) detector, the charge is moved to a charge-sensing amplifier for measurement. • Charge-coupled devices with front-end image intensifiers (ICCDs) can be gated on and off at selected intervals to provide time resolution for lifetime studies, for chemical kinetics, or to discriminate against undesirable signals. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 73 23A-4 Detecting and Measuring Radiant Energy (25 of 27) • Because photons in the infrared (IR) region lack sufficient energy to cause photoemission of electrons, convenient photon detectors cannot be used. • Currently, most Fourier transform IR spectrometers use a pyroelectric transducer or a mercury cadmium telluride (MCT) photoconductive detector. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 74 23A-4 Detecting and Measuring Radiant Energy (26 of 27) • A thermal detector consists of a tiny blackened surface that absorbs IR radiation and therefore increases in temperature. The temperature rise is converted to an electrical signal that is amplified and measured. • To minimize effects of background radiation, or noise, thermal detectors are housed in a vacuum and shielded; a rotating slotted disc called a chopper forces the beam to alternate between maximum and zero intensity. • The transducer converts this periodic radiation to an alternating electrical current that can be amplified and separated from the background dc signal. • However, IR measurements are less precise than measurements of UV and visible radiation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 75 23A-4 Detecting and Measuring Radiant Energy (27 of 27) • There are four types of thermal transducers used for IR spectroscopy: 1. The thermopile is a tiny thermocouple or group of thermocouples. 2. The bolometer is a conducting element whose electrical resistance changes as a function of temperature. 3. A pneumatic detector consists of a small cylindrical chamber that is filled with xenon and contains a blackened membrane to absorb IR radiation and heat the gas. 4. Pyroelectric detectors are manufactured from crystals of a pyroelectric material so that a crystal is sandwiched between a pair of electrodes and produces a temperature-dependent voltage when exposed to IR radiation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 76 23A-5 Signal Processors and Readout Devices • • A signal processor is an electronic device that may amplify the electrical signal from the detector convert the signal from ac to dc (or the reverse) change the phase of the signal filter the signal to remove unwanted components perform mathematical operations on the signal Examples of readout devices in modern instruments include digital meters and computer monitors. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 77 Feature 23-6: Measuring Photocurrents with Operational Amplifiers (1 of 2) The current produced by a reverse-biased silicon photodiode is typically 0.1 μA to 100 μA. These currents, as well as those generated by photomultipliers and phototubes, are so small that they must be converted to a voltage that is large enough to be measured with a digital voltmeter or other voltage-measuring device. We can perform such a conversion with the operational amplifier (op amp) circuit shown in Figure 23F-5. Light striking the reverse-biased photodiode causes a current I in the circuit. Because the op amp has a very large input resistance, essentially no current enters the op amp input designated by the minus sign. Thus, current in the photodiode must pass through the resistor R. The current is conveniently calculated from Ohm’s law: Eout IR. Since the current is proportional to the radiant power (P) of the light striking the photodiode, I = kP, where k is a constant, and therefore, Eout IR kPR k P . A voltmeter is connected to the output of the op amp to give a direct readout that is proportional to the radiant power of the light falling on the photodiode. This same circuit can also be used with vacuum photodiodes or photomultipliers. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 78 Feature 23-6: Measuring Photocurrents with Operational Amplifiers (2 of 2) Figure 23F-5 An operational amplifier current to voltage converter used to monitor the current in a solid-state photodiode. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 79 23A-6 Sample Containers (1 of 3) • Sample containers are usually called cells or cuvettes. • They must have windows that are transparent in the spectral region of interest. • Quartz or fused silica is required for the UV region and may be used in the visible region and out to about 3000 nm. Silicate glass is usually used for the 375 to 2000 nm region. The most common window region for IR studies is crystalline sodium chloride. The best cells have regions that are perpendicular to the direction of the beam to minimize reflection losses. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 80 23A-6 Sample Containers (2 of 3) • It is imperative to thoroughly clean cells before and after use because the transmission characteristics of the cell may be significantly altered by deposits on the cell walls. • Matched cells should never be dried by heating in an oven or over a flame as this may cause physical damage or change the path length. • Matched cells should be calibrated against each other regularly with an absorbing solution. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 81 23A-6 Sample Containers (3 of 3) • Figure 23-18 shows typical examples of cells for the UV/visible region. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 82 23B Ultraviolet/Visible Photometers and Spectrophotometers (1 of 2) • A spectrometer is a spectroscopic instrument that uses a monochromator or polychromator in conjunction with a transducer to convert radiant intensities into chemical signals. • Spectrophotometers are spectrometers that allow measurement of the ratio of the radiant powers of two beams (required to measure absorbance). • Photometers use a filter for wavelength selection in conjunction with a suitable radiation transducer. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 83 23B Ultraviolet/Visible Photometers and Spectrophotometers (2 of 2) • Advantages of specific devices are as follows: Spectrophotometers allow the wavelength to be varied continuously, allowing absorption spectra to be recorded. Photometers are simple, rugged, and inexpensive. • Spectrophotometers usually cover the UV/visible and occasionally the near-infrared region. • Photometers are most often used for the visible region. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 84 23B-1 Single-Beam Instruments (1 of 4) • Spectronic series instruments are commonly used spectrophotometers. • The Spectronic 20 is equipped with an occluder, which is a vane that automatically falls between the beam and the detector whenever the cylindrical cell is removed from its holder. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 85 23B-1 Single-Beam Instruments (2 of 4) • To obtain a percent transmittance reading using the Spectronic 20: 1. First perform the 0% T calibration or adjustment, in which the digital reading is zeroed out with the sample compartment empty so that the occluder blocks the beam. 2. Perform the 100% T calibration or adjustment, in which a cell containing the blank (often the solvent) is inserted into the cell holder and the pointer is brought to the 100% T mark by adjusting the position of the light control aperture. 3. Place the sample in the cell compartment and read the percent transmittance or absorbance from the LCD display. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 86 23B-1 Single-Beam Instruments (3 of 4) • The Spectronic 20 has been replaced by the Spectronic 200, which has features such as a wider spectral range, a spectral bandwidth of 4 nm instead of 20 nm, and the ability to accommodate square cuvettes. • The Spectronic 200 measures 0% T automatically at start-up and can record 100% T over its entire wavelength range. • A spectral scanning mode allows entire spectra to be recorded. • An emulation mode provides many of the same operations as legacy instruments so that methods and procedures developed previously can still be used. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 87 23B-1 Single-Beam Instruments (4 of 4) • When using single-beam instruments, the 0% T and 100% T adjustments should be made immediately before each transmittance or absorbance measurement. • To obtain reproducible transmittance measurements, the radiant power of the source must remain constant during the time that the 100% T adjustment is made and the % T is displayed. • Single-beam instruments are well-suited for quantitative absorption measurements at a single wavelength. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 88 23B-2 Double-Beam Instruments (1 of 4) • Figure 23-20 compares a single-beam system (a) with two double-beam designs (b and c). • Figure 23-20a shows a single-beam instrument in which radiation from the filter or monochromator passes through either the reference cell or the sample cell before striking the photodetector. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 89 23B-2 Double-Beam Instruments (2 of 4) • Figure 23-20b shows a double-beam-in-space instrument in which two beams are formed by a V-shaped mirror called a beam-splitter. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 90 23B-2 Double-Beam Instruments (3 of 4) Figure 23-20c shows a double-beam-in-time instrument in which the beams are separated in time by a rotating sector mirror. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 91 23B-2 Double-Beam Instruments (4 of 4) • Advantages of double-beam instruments are that they compensate for all but the most rapid fluctuations in radiant output of the source. compensate for wide variations of source intensity with wavelength. are well-suited for continuous recording of absorption spectra. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 92 23B-3 Multichannel Instruments (1 of 3) • Photodiode arrays and charge-transfer devices are the basis of multichannel instruments for UV-visible absorption. • The dispersive system is a grating spectrograph placed after the sample or reference cell. • The photodiode array or CCD array is placed in the focal plane of the spectrograph. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 93 23B-3 Multichannel Instruments (2 of 3) • With single-beam designs, the array dark current is acquired and stored in computer memory. • The raw spectrum of the sample is obtained, and, after dark current subtraction, the sample values are divided by the source values at each wavelength to produce the absorption spectrum. • Multichannel instruments can also be configured as double-beamin-time spectrophotometers. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 94 23B-3 Multichannel Instruments (3 of 3) • Figure 23-21 shows the most common, single-beam design. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 95 23C-1 Dispersive Infrared Instruments (1 of 2) • In most UV/visible instruments, the cell is located between the monochromator and the detector to avoid photodecomposition of the sample. • Photodiode-array instruments avoid this problem because of the short exposure time of the sample to the beam. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 96 23C-1 Dispersive Infrared Instruments (2 of 2) • Older IR instruments were almost always dispersive double-beam designs, often of the double-beam-in-time variety except that the location of the cell compartment with respect to the monochromator was reversed. • In IR instruments, the cell compartment is usually located between the source and the monochromator. IR is not sufficiently energetic to bring about photodecomposition. Most samples are good emitters of IR radiation. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 97 23C-2 Fourier Transform Instruments (1 of 4) • Fourier transform infrared (FTIR) spectrometers have replaced dispersive instruments in most laboratories. • FTIR transform spectrophotometers detect all IR wavelengths all the time. They have greater light-gathering power than dispersive instruments and consequently better precision. • The complex calculations required are easily accomplished with appropriate computers and software. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 98 23C-2 Fourier Transform Instruments (2 of 4) • FTIR spectrometers contain no dispersing element. • An interferometer is used to produce interference patterns that contain IR spectral information (instead of using a monochromator). • The interferometer modulates the source signal so it can be decoded by the mathematical technique of Fourier transformation. • Most benchtop FTIR spectrophotometers are of the single-beam type. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 99 23C-2 Fourier Transform Instruments (3 of 4) • To collect the spectrum of a sample: 1. The background spectrum is obtained by Fourier transformation of the interferogram from the background. 2. The sample spectrum is acquired. 3. The ratio of the single-beam spectrum to that of the background spectrum is calculated and the absorbance or transmittance versus wavelength or wavenumber are plotted. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 100 23C-2 Fourier Transform Instruments (4 of 4) • Major advantages of FTIR instruments over dispersive spectrophotometers include better speed and sensitivity better light-gathering power more accurate wavelength calibration simpler mechanical design virtual elimination of any contribution from stray light and IR emission Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 101 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (1 of 14) Fourier transform infrared (FTIR) spectrometers utilize an ingenious device called a Michelson interferometer, which was developed many years ago by A. A. Michelson for making precise measurements of the wavelengths of electromagnetic radiation and for making incredibly accurate distance measurements. The principles of interferometry are utilized in many areas of science including chemistry, physics, astronomy, and metrology and are applicable in many regions of the electromagnetic spectrum. A diagram of a Michelson interferometer is shown in Figure 23F-6. It consists of a collimated light source, shown on the left of the diagram; a stationary mirror at the top; a moveable mirror at the right; a beam-splitter; and a detector. The light source may be a continuum source as in FTIR spectroscopy, or it may be a monochromatic source such as a laser or a sodium arc lamp for other uses such as, for example, measuring distances. The mirrors are precision-polished ultraflat glass with a reflective coating vapor deposited on their front surfaces. The moveable mirror is usually mounted on a very precise linear bearing that allows it to move along the direction of the light beam while remaining perpendicular to it as shown in the diagram. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 102 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (2 of 14) Figure 23F-6 Diagram of a Michelson interferometer. A beam from the light source on left is split into two beams by the beam-splitter. The two beams travel two separate paths and converge on the detector. The two beams A′ and B converge in the same region of space and form an interference pattern. As the movable mirror on the right is moved, the interference pattern shifts across the detector and modulates the optical signal. The resulting reference interferogram is recorded and used as a measure of the power of the incident beam at all wavelengths. An absorbing sample is then inserted into the beam, and a sample interferogram is recorded. The two interferograms are then used to compute the absorption spectrum of the sample. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 103 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (3 of 14) The key to the operation of the interferometer is the beamsplitter, which is usually a partially silvered mirror similar to the “two-way” mirrors often seen in retail stores and police interrogation rooms. The beam-splitter allows a fraction of the light falling on it to pass through the mirror, and another fraction is reflected. This device works in both directions so that light falling on either side of the beam-splitter is partially reflected and partially transmitted. For simplicity, we will use as our light source the blue line of an argon-ion laser. Beam A from the source impinges on the beam-splitter, which is tilted at 45° to the incoming beam. Our beamsplitter is coated on the right side, so Beam A enters the glass and is partially reflected off the back side of the coating. It emerges from the beam-splitter as Beam A′ and moves up toward the stationary mirror where it is reflected back down toward the beam-splitter. Part of the beam is then transmitted down through the beam-splitter toward the detector. Although the beam loses some intensity with each interaction with the stationary mirror and the beam-splitter, the net effect is that a fraction (Beam A′) of incident Beam A ends up at the detector. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 104 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (4 of 14) In its first interaction with the beam-splitter, the fraction of Beam A that is transmitted emerges to the right toward the moveable mirror as Beam B. It then is reflected back to the left to the beam-splitter where it is reflected down toward the detector. With careful alignment, both Beam A′ and Beam B (shown separately in the diagram for clarity) are collinear and impinge on the detector at the same spot. The overall purpose of the interferometer optics is to split the incident beam into two beams that move through space along separate paths and then recombine at the detector. It is in this region that the two beams, or wavefronts, interact to form an interference pattern. The origin of the interference pattern is illustrated in Figure 23F-7, which is a two-dimensional representation of the interaction of the two spherical wavefronts. Beam A′ and Beam B converge and interact as two point sources of light represented in the upper portion of the figure. When the two beams interfere, they form a pattern similar to the one shown. In regions where the waves interfere constructively, bright bands appear, and where destructive interference occurs, dark bands form. The alternating light and dark bands are called interference fringes. These fringes appear at the detector as the output image shown at the bottom of the figure. In the earliest versions of the Michelson interferometer, the detector was the human eye aided by a telescope. The fringes could be counted or measured through the telescope. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 105 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (5 of 14) Figure 23F-7 A two-dimensional representation of the interference of two monochromatic wavefronts of the same frequency. Beam A′ and Beam B at the top form the interference pattern in the middle, and the two wavefronts constructively and destructively interfere. The image shown at the bottom would appear at the output of the Michelson interferometer perpendicular to the plane of the two-dimensional interference pattern. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 106 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (6 of 14) When the moveable mirror is moved to the left at constant velocity, the interference pattern gradually sweeps past the detector as the path that Beam B follows is gradually shortened. The form of the interference pattern remains the same, but the positions of constructive and destructive interference are shifted as the path difference changes. For example, if the wavelength of our laser source is λ, as we move the mirror a distance of λ 4, the path difference between the two beams changes by λ 2, and where there was constructive interference, now there is destructive interference. If we move the mirror another λ 4, the path difference changes again by λ 2, and we again return to constructive interference. As the mirror moves, the two wavefronts are shifted in space relative to one another, and alternate light and dark fringes sweep across the detector, as illustrated in Figure 23F-8a. At the detector, find the sinusoidal intensity profile shown in Figure 23F-8b. This profile is called an interferogram. The net effect of the constant uniform motion of the mirror is that the light intensity at the output of the interferometer is modulated, or systematically varied, in a precisely controlled way as shown in the figure. In practice, it turns out not to be very easy to move the interferometer mirror at a constant, precisely controlled velocity. There is a better and much more precise way to monitor the mirror motion using a second parallel interferometer. In this example, just assume that we can measure and/or monitor the progress of the mirror and compensate for any nonuniform motion computationally. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 107 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (7 of 14) Figure 23F-8 Formation of interferograms at the output of the Michelson interferometer. (a) Interference pattern at the output of the interferometer resulting from a monochromatic source. (b) Sinusoidally varying signal produced at the detector by the pattern in (a). (c) Frequency spectrum of the monochromatic light source resulting from the Fourier transformation of the signal in (b). (d) Interference pattern at the output of the interferometer resulting from a two-color source. (e) Complex signal produced by the interference pattern of (d) as it falls on the detector. (f) Frequency spectrum of the two-color source. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 108 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (8 of 14) We have established that a Michelson interferometer with a monochromatic light source produces a sinusoidally varying signal at the detector when the mirror is moved at constant velocity. Now, we must investigate what happens to the signal once it is recorded. Although the characteristics of Michelson interferometers have been well known for over a century and the mathematical apparatus for dealing with the data has been in place for nearly two centuries, the device could not be used routinely for spectroscopy until two developments occurred. First, high-speed, inexpensive computers had to become available. Second, appropriate computational methods had to be invented to handle the huge number of rather routine calculations that must be applied to the raw data acquired in interferometric experiments. Briefly, the principles of Fourier synthesis and analysis tell us that any waveform can be represented as a series of sinusoidal waveforms, and correspondingly, any combination of sinusoidal waveforms can be broken down into a series of sinusoids of known frequency. Apply this idea to the sinusoidal signal detected at the output of the Michelson interferometer shown in Figure 23F-8b. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 109 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (9 of 14) If we subject the signal in the figure to Fourier analysis via a computer algorithm called the fast Fourier transform (FFT), we obtain the frequency spectrum illustrated in Figure 23F-8c. Notice that the original waveform in Figure 23F-8b is a time-dependent signal; the resultant output from the FFT is a frequency-dependent signal. In other words, the FFT takes amplitude signals in the time domain and converts them to power in the frequency domain. Since the output of the interferometer is a sine wave of a single frequency, the frequency spectrum shows a single spike of frequency v, the frequency of the original sine wave. This frequency is proportional to the optical frequency emitted by the laser source but of much lower value so that it can be measured and manipulated electronically. In many instruments, the interferometer is modified to obtain a second sine wave at the output. One way to do this is to simply add a second wavelength to our light source. Experimentally, a second laser or another monochromatic light source at the input of the interferometer gives us a beam that contains just two wavelengths. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 110 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (10 of 14) For example, assume that the second wavelength is one quarter of the first one so that the second frequency is 4v. Further assume that its intensity is one half the intensity of the original source. As a result, the signal appearing at the output of the interferometer exhibits a pattern somewhat more complex than in the singlewavelength example, as shown in Figure 23F-8d. The detector signal plot appears as the sum of two sine waves as depicted in Figure 23F-8e. Then apply the FFT to the complex sinusoidal signal to produce the frequency spectrum of Figure 23F-8f. This spectrum reveals just two frequencies at v and 4v, and the relative magnitudes of the two frequency spikes are proportional to the amplitudes of the two sine waves composing the original signal. The two frequencies correspond to the two wavelengths in our interferometer light source, and the FFT has revealed the intensities of the source at those two wavelengths. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 111 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (11 of 14) To illustrate how the Michelson interferometer is used in practical experiments, place a continuum infrared light source (see Figure 23F-9a) containing a huge number of wavelengths at the input of the interferometer. As the mirror moves along its path, all wavelengths are modulated simultaneously, producing the very interesting interferogram shown in Figure 23F-9b. This interferogram contains all the information required in a spectroscopy experiment regarding the intensity of the light source at all its component wavelengths. As suggested in the previous section, there are a number of advantages to acquiring intensity information in this way rather than using a scanning spectrometer. First, there is the advantage of speed. The mirror can be moved in a matter of seconds, and a computer attached to the detector can collect all necessary data during the course of the mirror scan. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 112 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (12 of 14) Figure 23F-9 (a) Spectrum of a continuum light source. (b) Interferogram of the light source in (a) produced at the output of the Michelson interferometer. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 113 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (13 of 14) In just a few more seconds, the computer can perform the FFT and produce the frequency spectrum containing all the intensity information. Next is Fellgett’s advantage, which suggests that Michelson interferometers are capable of producing higher signal-to-noise ratios in shorter time than equivalent dispersive spectrometers. Finally, we have the throughput, or Jacquinot’s advantage, which permits 10 to 200 times more radiation to pass through a sample compared to standard dispersive spectrometers, which are limited by entrance and exit slits. These advantages are often partially offset by the lower sensitivity of detectors that are used in FTIR spectrometers. Under these circumstances, the speed of the measurement process and the simplicity and reliability of FTIR spectrometers become primary considerations. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 114 Feature 23-7: How Does a Fourier Transform Infrared Spectrophotometer Work? (14 of 14) Up to this point in our discussion of the FTIR spectrometer, we have shown how the Michelson interferometer can provide intensity information for a light source as a function of wavelength. To collect the IR spectrum of a sample, we must first obtain a reference interferogram of the source with no sample in the light path, as shown in Figure 23F-6. Then, the sample is placed in the path as indicated by the arrow and dashed box in the figure, and once again, we scan the mirror and acquire a second interferogram. In FTIR spectrometry, the sample absorbs infrared radiation, which attenuates the beams in the interferometer. The difference between the second (sample) interferogram and the reference interferogram is then computed. Since the difference in interferogram depends only on the absorption of radiation by the sample, the FFT is performed on the resulting data, which produces the IR spectrum of the sample. We discuss a specific example of this process in Chapter 24. Finally, note that the FFT can be accomplished using the most basic modern personal computer equipped with the appropriate software. Many software packages such as Mathcad, Mathematica, Matlab, and even the Data Analysis Toolpak of Microsoft Excel have Fourier analysis functions built in. These tools are widely used in science and engineering for a broad range of signal-processing tasks. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 115 Analytical Chemistry Online Activity Search for companies that manufacture monochromators. Navigate to websites of these companies, and find a UV/visible monochromator of the Czerny-Turner design that has better than 0.1 nm resolution. List several other important specifications of monochromators, and describe what they mean and how they affect the quality of analytical spectroscopic measurements. From the specifications and, if available, prices, determine the factors that have the most significant effect on the cost of the monochromators. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 116 Key Terms Activity (1 of 2) • Angular dispersion • Echellete grating • Charge-transfer device • Effective bandwidth • Continuum source • Excimer laser • Dark current • Fellgett’s advantage • Detector • Focal plane • Diode array • Gas laser • Dynode • Holographic grating • Echelle grating • Interference filter Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 117 Key Terms Activity (2 of 2) • Interferogram • Reciprocal linear dispersion • Jacquinot’s advantage • Singlet state • Michelson interferometer • Spectral bandpass • Monochromator • Spectrograph • Nernst glower • Thermopile • Photocurrent • Transducer • Polychromator Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 118 Assessments: Discussion Questions (1 of 2) 23-1. Describe the differences between the following pairs of terms, and list any particular advantages possessed by one over the other: a) solid-state photodiodes and phototubes as detectors for electromagnetic radiation. b) phototubes and photomultiplier tubes. c) filters and monochromators as wavelength selectors. d) conventional and diode-array spectrophotometers. 22-3. Why do quantitative and qualitative analyses often require different monochromator slit widths? Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 119 Assessments: Discussion Questions (2 of 2) 23-4. Why are photomultiplier tubes unsuited for the detection of infrared radiation? 23-5. Why is iodine sometimes introduced into a tungsten lamp? Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 120 Challenge Problem (1 of 4) 23-24. Challenge Problem: Horlick has described principles of the Fourier transform, interpreted them graphically, and described how they may be used in analytical spectroscopy. Read the article and answer the following questions. (a) Define time domain and frequency domain. (b) Write the equations for the Fourier integral and its transformation, and define each of the terms in the equation. G. Horlick, Anal. Chem., 1971, 43(8), 61A-66A, DOI: 10.1021/ac60303a029. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 121 Challenge Problem (2 of 4) (c) The paper shows the time-domain signals for a 32-cycle cosine wave, a 21cycle cosine wave, and a 10-cycle cosine wave as well as the Fourier transforms of these signals. How does the shape of the frequency-domain signal change as the number of cycles in the original waveform changes? (d) The author describes the phenomenon of damping. What effect does damping have on the original cosine waves? What effect does it have on the resulting Fourier transformations? (e) What is a resolution function? (f) What is the process of convolution? (g) Discuss how the choice of the resolution function can affect the appearance of a spectrum. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 122 Challenge Problem (3 of 4) (h) Convolution may be used to decrease the amount of noise in a noisy spectrum. Consider the following plots of time-domain and frequency domain signals. Label the axes of the five plots. For example, (b) should be labeled as amplitude versus time. Characterize each plot as either a time-domain or a frequencydomain signal. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 123 Challenge Problem (4 of 4) (i) Describe the mathematical relationships among the plots. For example, how could you arrive at (a) from (d) and (e)? (j) Discuss the practical importance of being able to reduce noise in spectroscopic signals. Skoog, West, Holler, and Crouch, Fundamentals of Analytical Chemistry, 10e. © 2022 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 124