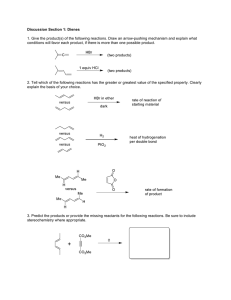
Problems for Chapter 34 PROBLEM 1 Predict the structure of the product of this Diels-Alder reaction. PROBLEM 2 Comment on the difference in rate between these two reactions. PROBLEM 3 Justify the stereoselectivity in this intramolecular Diels-Alder reaction. 34 140 Problems to accompany Organic Chemistry PROBLEM 4 Explain the formation of single adducts in these reactions. PROBLEM 5 Suggest two syntheses of this spirocyclic ketone from the starting materials shown. Neither starting material is available. PROBLEM 6 Draw mechanisms for these reactions and explain the stereochemistry. Problems for Chapter 34 – Pericyclic reactions 1: cycloadditions PROBLEM 7 Give mechanisms for these reactions and explain the regio- and stereochemical control (or lack of it!). Note that MnO2 oxidizes allylic alcohols to enones. PROBLEM 8 Suggest a mechanism for this reaction and explain the stereo- and regiochemistry. PROBLEM 9 Photochemical cycloaddition of these two compounds is claimed to give the diastereoisomer shown. The chemists who did this work claimed that the stereochemistry of the adduct is simply proved by its conversion into a lactone on reduction. Comment on the validity of this deduction and explain the stereochemistry of the cycloaddition. 141 142 Problems to accompany Organic Chemistry PROBLEM 10 Thioketones, with a C=S bond, are not usually stable. However, this thioketone is quite stable and undergoes reaction with maleic anhydride to give an addition product. Comment on the stability of the thioketone, the mechanism of the reaction, and the stereochemistry of the product. PROBLEM 11 This unsaturated alcohol is perfectly stable until it is oxidized with Cr(VI): it then cyclizes to the product shown. Explain. PROBLEM 12 Give mechanisms for these reactions, explaining the stereochemistry.