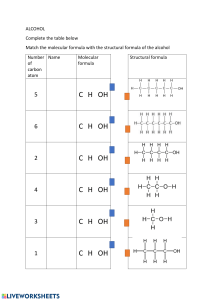
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai’i Chapter 13 Mass Spectrometry and Infrared Spectroscopy Prepared by Rabi Ann Musah State University of New York at Albany Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Mass Spectrometry 13.1 Mass Spectrometry • Mass spectrometry is a technique used for measuring the molecular weight and determining the molecular formula of an organic compound. • In a mass spectrometer, a molecule is vaporized and ionized by bombardment with a beam of high-energy electrons. • The energy of the electrons is ~ 1600 kcal (or 70 eV). • Since it takes ~100 kcal of energy to cleave a typical σ bond, 1600 kcal is an enormous amount of energy to come into contact with a molecule. • The electron beam ionizes the molecule by causing it to eject an electron. 2 1 Figure 13.1 Schematic of a mass spectrometer 3 • When the electron beam ionizes the molecule, the species that is formed is called a radical cation, and symbolized as M+•. • The radical cation M+• is called the molecular ion or parent ion. • The mass of M+• represents the molecular weight of M. • Because M is unstable, it decomposes to form fragments of radicals and cations that have a lower molecular weight than M+•. • The mass spectrometer analyzes the masses of cations. • A mass spectrum is a plot of the amount of each cation (its relative abundance) versus its mass to charge ratio (m/z, where m is mass, and z is charge). • Since z is almost always +1, m/z actually measures the mass (m) of the individual ions. 4 2 Consider the mass spectrum of CH4 below: • The tallest peak in the mass spectrum is called the base peak. • The base peak is also the M peak, although this may not always be the case. • Though most C atoms have an atomic mass of 12, 1.1% have a mass of 13. Thus, 13CH4 is responsible for the peak at m/z = 17. This is called the M + 1 peak. 5 • The mass spectrum of CH4 consists of more peaks than just the M peak. • Since the molecular ion is unstable, it fragments into other cations and radical cations containing one, two, three, or four fewer hydrogen atoms than methane itself. • Thus, the peaks at m/z 15, 14, 13 and 12 are due to these lower molecular weight fragments. 6 3 Figure 13.2 Mass spectrum of hexane (CH3CH2CH2CH2CH2CH3) 7 The Nitrogen Rule • Hydrocarbons like methane (CH4) and hexane (C6H14), as well as compounds that contain only C, H, and O atoms, always have a molecular ion with an even mass. • An odd molecular ion indicates that a compound has an odd number of nitrogen atoms. • The effect of N atoms on the mass of the molecular ion in a mass spectrum is called the nitrogen rule: A compound that contains an odd number of N atoms gives an odd molecular ion. A compound that contains an even number of N atoms (including zero) gives an even molecular ion. • Two “street” drugs that mimic the effect of heroin illustrate this principle. 8 4 13.2 Alkyl Halides and the M + 2 Peak • Most elements have one major isotope. • Chlorine has two common isotopes, 35Cl and 37Cl, which occur naturally in a 3:1 ratio. Thus, there are two peaks in a 3:1 ratio for the molecular ion of an alkyl chloride. The larger peak, the M peak, corresponds to the compound containing the 35Cl. The smaller peak, the M + 2 peak, corresponds to the compound containing 37Cl. Thus, when the molecular ion consists of two peaks (M and M + 2) in a 3:1 ratio, a Cl atom is present. • Br has two isotopes—79Br and 81Br, in a ratio of ~1:1. Thus, when the molecular ion consists of two peaks (M and M + 2) in a 1:1 ratio, a Br atom is present. 9 Figure 13.3 Mass spectrum of 2-chloropropane [(CH3)2CHCI] 10 5 Figure 13.4 Mass spectrum of 2-bromopropane [(CH3)2CHBr] 11 13.3 Other Types of Mass Spectrometry 13.3A High Resolution Mass Spectrometers • Low resolution mass spectrometers report m/z values to the nearest whole number. Thus, the mass of a given molecular ion can correspond to many different molecular formulas. • High resolution mass spectrometers measure m/z ratios to four (or more) decimal places. This is valuable because except for 12C whose mass is defined as 12.0000, the masses of all other nuclei are very close—but not exactly—whole numbers. Table 13.1 lists the exact mass values for a few common nuclei. Using these values it is possible to determine the single molecular formula that gives rise to a molecular ion. 12 6 High-Resolution Mass Spectrometers • Consider a compound having a molecular ion at m/z = 60 using a lowresolution mass spectrometer. The molecule could have any one of the following molecular formulas. 13 13.3B Gas Chromatography—Mass Spectrometry (GC-MS) • Mass spectrometry can be combined with gas chromatography into a single instrument used to analyze mixtures of compounds. • A gas chromatograph (GC) consists of a thin capillary column containing a viscous high-boiling liquid, all housed in an oven. When the sample is injected into the GC, it is vaporized and swept by an inert gas through the column. • The components travel through the column at different rates, often separated by boiling point, with lower boiling compounds exiting the column before higher boiling compounds. • Each component then enters the mass spectrometer where it is ionized to form a molecular ion and lower molecular weight fragments • The GC-MS records a gas chromatogram of the mixture, and yields a plot of the amount of each component versus its retention time (the time required to travel through the column), and its molecular ion in the mass spectrum. 14 7 Figure 13.5 Compound analysis using GC–MS 15 • To analyze a urine sample for tetrahydrocannabinol, (THC) the principal psychoactive component of marijuana, the organic compounds are extracted from urine, purified, concentrated and injected into the GC-MS. • THC appears as a GC peak, and gives a molecular ion at 314, its molecular weight. Figure 13.6 Mass spectrum of tetrahydrocannabinol (THC) 16 8 Infrared Spectroscopy 13.4 Electromagnetic Radiation • Electromagnetic radiation is radiant energy having dual properties of both waves and particles. • Particles of electromagnetic radiation are called photons, and each has a discrete amount of energy called a quantum. • Electromagnetic radiation can be characterized by its wavelength and frequency. • Wavelength (λ) is the distance from one point on a wave to the same point on an adjacent wave. • Frequency (ν) is the number of waves passing per unit time. It is reported in cycles per second (s-1), which is also called hertz (Hz). 17 • The different forms of electromagnetic spectrum. electromagnetic radiation make up the Figure 13.7 The electromagnetic spectrum 18 9 • The electromagnetic spectrum is arbitrarily divided into different regions. • All electromagnetic radiation travels at the speed of light (c), 3.0 x 108 m/s. • The speed of electromagnetic radiation (c) is directly proportional to its wavelength and frequency: c = λν • The speed of light (c) is a constant, so wavelength and frequency are inversely related: λ = c/ν : Wavelength increases as frequency decreases. ν = c/λ : Frequency increases as wavelength decreases. • The energy (E) of a photon is directly proportional to its frequency: E = hν ; h = Planck’s constant (1.58 x 10-34 cal•s) 19 • Frequency and wavelength are inversely proportional (ν = c/λ). Thus, energy and wavelength are inversely proportional. E = hν = hc/λ •E increases as ν increases •E decreases as λ increases • When electromagnetic radiation strikes a molecule, some wavelengths, but not all, are absorbed. • For absorption to occur, the energy of the photon must match the difference between two energy states in the molecule. • The larger the energy difference between two states, the higher the energy of radiation needed for absorption, the higher the frequency, and the shorter the wavelength. 20 10 13.5 Infrared Spectroscopy 13.5A Background • Infrared (IR) spectroscopy is used to identify the functional groups in a compound. • IR radiation is the energy source used in IR spectroscopy. • Frequencies in IR spectroscopy are reported using a unit called ~ wavenumber (ν): ~ ν = 1/λ • Wavenumber is inversely proportional to wavelength and is reported in reciprocal centimeters (cm–1). • Frequency (and therefore, energy) increases as the wavenumber increases. • Using the wavenumber scale, IR absorptions occur from 4000 cm–1– 400 cm–1. 21 • Absorption of IR light causes changes in the vibrational motions of a molecule. • The different vibrational modes available to a molecule include stretching and bending modes. • The vibrational modes of a molecule are quantized, so they occur only at specific frequencies which correspond to the frequency of IR light. 22 11 • When the frequency of IR light matches the frequency of a particular vibrational mode, the IR light is absorbed, causing the amplitude of the particular bond stretch or bond bend to increase. 23 13.5B Characteristics of an IR Spectrum • In an IR spectrometer, light passes through a sample. • Frequencies that match the vibrational frequencies are absorbed, and the remaining light is transmitted to a detector. • An IR spectrum is a plot of the amount of transmitted light versus its wavenumber. Let us now consider the IR spectrum of 1-propanol, CH3CH2CH2OH. 24 12 Characteristics of an IR Spectrum—1-Propanol 25 • The y-axis is % transmittance: 100% transmittance means that all the light shone on a sample is transmitted and none is absorbed. 0% transmittance means that none of the light shone on the sample is transmitted and all is absorbed. • Each peak corresponds to a particular kind of bond, and each bond type (such as O—H and C—H) occurs at a characteristic frequency. • Wavenumber, frequency and energy decrease from left to right. • Where a peak occurs is reported in reciprocal centimeters (cm-1). 26 13 • The IR spectrum is divided into two regions: the functional group region (at ≥ 1500 cm-1), and the fingerprint region (at < 1500 cm-1). Figure 13.8 Comparing the functional group region and fingerprint region of two compounds 27 13.6 IR Absorptions • Where a particular bond absorbs in the IR depends on bond strength and atom mass. • Stronger bonds vibrate at a higher frequency, so they absorb at higher wavenumbers. • Bonds with lighter atoms vibrate at higher frequency, so they absorb at higher wavenumbers. • Bonds can be thought of as springs with weights on each end. • The strength of the spring is analogous to the bond strength, and the mass of the weights is analogous to atomic mass. • For two springs with the same weight on each end, the stronger spring vibrates at a higher frequency. • For two springs of the same strength, springs with lighter weights vibrate at a higher frequency than those with heavier weights. 28 14 • Hooke’s Law describes the relationship of frequency to mass and bond length. Figure 13.9 Hook’s law: How the frequency of bond vibration depends on atom mass and bond strength 29 • Bonds absorb in four predictable regions of an IR spectrum. Figure 13.10 Summary: The four regions of the IR spectrum 30 15 31 • Even subtle differences that affect bond strength affect the frequency of an IR absorption. • The higher the percent s-character, the stronger the bond and the higher the wavenumber of absorption. 32 16 • For a bond to absorb in the IR, there must be a change in dipole moment during the vibration. • Symmetrical nonpolar bonds do not absorb in the IR. This type of vibration is said to be IR inactive. 33 13.6B IR Absorptions in Hydrocarbons Hexane has only C-C single bonds and sp3 hybridized C atoms. Therefore it has only one major absorption at 3000-2850 cm-1. 34 17 1-Hexene has a C=C and Csp2-H, in addition to sp3 hybridized C atoms. cm-1; Therefore, there are three major absorptions: Csp2-H at 3150-3000 Csp3-H at 3000-2850 cm-1; C=C at 1650 cm-1. 35 1-Hexyne has a C≡C and Csp-H, in addition to sp3 hybridized C atoms. Therefore, there are three major absorptions: Csp-H at 3300 cm-1; Csp3-H at 3000-2850 cm-1; C≡C at 2250 cm-1. 36 18 13.6C IR Absorptions in Oxygen Containing Compounds The OH group of the alcohol shows a strong absorption at 3600-3200 cm-1. The peak at ~3000 cm-1 is due to sp3 hybridized C—H bonds. 37 The C=O group in the ketone shows a strong absorption at ~1700 cm-1. The peak at ~3000 cm-1 is due to sp3 hybridized C—H bonds. 38 19 The ether has neither an OH or a C=O, so its only absorption above 1500 cm-1 occurs at ~3000 cm-1, due to sp3 hybridized C—H bonds. 39 13.6D IR Absorptions in Nitrogen Containing Compounds The N—H bonds in the amine give rise to two weak absorptions at 3300 and 3400 cm-1. 40 20 The amide exhibits absorptions above 1500 cm-1 for both its N—H and C=O groups: N—H (two peaks) at 3200 and 3400 cm-1; C=O at 1660 cm-1. 41 The C≡N of the nitrile absorbs in the triple bond region at ~2250 cm-1. 42 21 13.7 IR and Structure Determination IR spectroscopy is often used to determine the outcome of a chemical reaction. For example, oxidation of the hydroxy group in compound C to form the carbonyl group in periplanone B is accompanied by the disappearance of the OH absorption, and the appearance of a carbonyl absorption in the IR spectrum of the product. 43 44 22 45 23