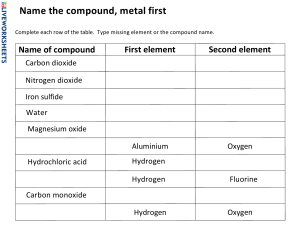
MID-TERM REVISION Unit 2 : Materials and their structure HOW ARE THE PARTICLES ARRANGED IN A SOLID, LIQUID, AND GAS? •In a solid, particles are tightly packed and do not move much. In a liquid, particles are close but can move around each other. In a gas, particles are far apart and move freely. WHY DO GASES SPREAD OUT TO FILL THE ENTIRE SPACE IN A CONTAINER? The particles in a gas move quickly in all directions, so they spread out to fill the space. WHAT IS THE DIFFERENCE BETWEEN BOILING AND EVAPORATION? •Boiling happens throughout the liquid at a specific temperature, while evaporation only happens at the surface of the liquid and at any temperature. WHAT IS SUBLIMATION? CAN YOU GIVE AN EXAMPLE? •Sublimation is when a solid changes directly into a gas without becoming a liquid first. An example is dry ice turning into carbon dioxide gas. YOU HAVE A BALLOON FILLED WITH AIR. IF YOU PUT IT IN THE FREEZER, WHAT WILL HAPPEN TO THE BALLOON? WHY? •The balloon will shrink because the cold temperature causes the gas molecules to move more slowly and come closer together, taking up less space. HOW DOES THE WATER CYCLE AFFECT THE AMOUNT OF FRESH WATER AVAILABLE TO US? •The water cycle continually moves water through evaporation, condensation, and precipitation, making fresh water available to lakes, rivers, and groundwater that we can use for drinking and farming. •\ IF A GOLD RING IS CUT INTO TINY PIECES, WILL THE PIECES STILL BE GOLD? WHY OR WHY NOT? •Yes, the pieces will still be gold because gold is an element. Each piece, no matter how small, is made up of gold atoms, which retain the properties of the element. HOW CAN WE EXPLAIN WHY WATER (H₂O) IS DIFFERENT FROM THE ELEMENTS HYDROGEN (H) AND OXYGEN (O) THAT MAKE IT UP? •Water is a compound formed when hydrogen and oxygen atoms chemically bond. Although water is made from hydrogen and oxygen, its properties (like being a liquid at room temperature) are very different from the gases hydrogen and oxygen because when atoms combine, they form new substances with different properties. IF YOU MIX TWO ELEMENTS TOGETHER, WILL THEY ALWAYS FORM A COMPOUND? •No, just mixing two elements together doesn’t guarantee a compound will form. For a compound to form, the elements must chemically bond. For example, mixing iron filings and sulfur will not create iron sulfide unless you heat them so they chemically react. WHY DO WE NEED TO KNOW THE CHEMICAL FORMULA OF COMPOUNDS? The chemical formula tells us which elements are in a compound and in what proportions. For example, the formula H₂O shows that water is made up of two hydrogen atoms and one oxygen atom. This helps us understand the composition of substances. WHY IS CARBON DIOXIDE (CO₂) CALLED A "DIOXIDE"? •The "di-" prefix means "two," and since carbon dioxide is made of one carbon atom and two oxygen atoms, it’s named carbon dioxide to reflect its composition. WHAT WOULD YOU NAME A COMPOUND MADE OF SODIUM (NA) AND SULFUR (S)? The compound would be named sodium sulfide because it follows the naming rule for ionic compounds: the name of the metal (sodium) comes first, followed by the non-metal (sulfur), with the "-ide" suffix added. WHY IS THE PERIODIC TABLE ORGANIZED IN ROWS AND COLUMNS? •The rows (called periods) represent increasing atomic numbers, while the columns (called groups) contain elements with similar chemical properties. This organization helps scientists easily predict how elements will behave in reactions based on their position. WHY ARE ELEMENTS LIKE HELIUM (HE) AND NEON (NE) PLACED IN THE SAME GROUP ON THE PERIODIC TABLE? •Helium and neon are both noble gases, meaning they are very stable and don’t usually react with other elements. Elements in the same group share similar chemical properties, which is why they are placed together. WHICH OF THE FOLLOWING IS A CHARACTERISTIC OF A MIXTURE? A) FIXED COMPOSITION B) CHEMICAL BONDS BETWEEN COMPONENTS C) COMPONENTS RETAIN THEIR INDIVIDUAL PROPERTIES D) UNIFORM COMPOSITION THROUGHOUT C)Components retain their individual properties WHICH OF THE FOLLOWING IS AN EXAMPLE OF A COMPOUND? A) AIR B) SALTWATER C) SODIUM CHLORIDE (TABLE SALT) D) SAND C) Sodium chloride (table salt) WHICH OF THE FOLLOWING IS AN EXAMPLE OF A COMPOUND? A) AIR B) SALTWATER C) SODIUM CHLORIDE (TABLE SALT) D) SAND C) Sodium chloride (table salt) WHICH OF THE FOLLOWING BEST DESCRIBES A COMPOUND? A) A SUBSTANCE THAT CAN BE PHYSICALLY SEPARATED INTO ITS COMPONENTS B) A SUBSTANCE WITH A FIXED RATIO OF ELEMENTS C) A MIXTURE WHERE COMPONENTS ARE IN DIFFERENT PHASES B) A substance with a fixed ratio of elements