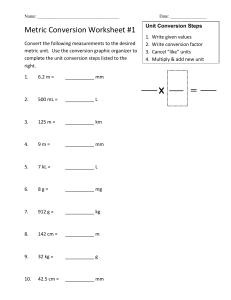
LESSON OBJECTIVES : Familiarize with different system of unit of measurements Convert units of measurements Solve measurements problems involving conversion of units LESSON 1 : Solve Measurement Problem Involving Conversion of Units WHAT IS PHYSICS ? MEASUREMENTS Where do we use measurement ? MEASUREMENT Is the assignment of numerical value to an object’s physical properties We need to assign units to numerical quantity of measurements to convey the relative size of magnitude of property 2 SYSTEMS OF UNITS METRIC SYSTEM Has 2 variations ; cgs and mks system. The International System of Units ( SI system) from French Le System International d’ Unites , is the modern form of Metric System Metric or SI is more convenient to use 2. English System Makes use of fps system which is the abbreviation of the fundamental units which are feet, pounds, seconds it does not use decimalization and it’s not a multiple of 10. DERIVED QUANTITIES Are quantities resulting from the combination of any of the fundamental quantities. Conversion of Units CONVERSION UNIT – a ratio expressing how many of one unit are equal to another unit. Steps in Unit Conversion: Identify the value to be converted. Find the conversion factors that can be used to change the units. Multiply the original value to the conversion factor too eliminate and change the units. Write the equivalence SAMPLE PROBLEM : ( metric system) 1.1024 mm = ________ m 2.1,673 mg = _______ kg 3.35.8 km = _______ dam 4.0.46 g = ________ cg 5.2.6 kL= _________ L EXERCISES : 1. 396 m to ___________km 2. 60 miles to feet 3. 47 cm to _____ inches 4. 8.65 yrs. to _____ sec 5. 12 m2 to _______ cm2 I. Metric to Metric Conversion SAMPLE PROBLEM : Nanotechnology is a term that embraces the use of materials of size in the order of a nanometer. What is 2.0 nanometer in (a) meters and (b) centimeters? SOLUTION: A. First form the conversion ratio or factor with the meter at the numerator and the nanometer at the denominator. 1 nm = 10 −9 m. Therefore our conversion ratio is Multiply the original quantity by this conversion ratio 2.0 nm x = 2.0 x 10 ¯ 9 m 10 − 9 1 nm B. To convert to cm, multiply the answer in letter a by the factor 2.0 x 10¯9 m x 1 cm 10¯2 m = 2.0 x 10¯7 cm II. Metric to English Conversion SAMPLE PROBLEM : How many pounds of chocolate are there in 2.3 kg ? First form the conversion ratio or factor with the pound at the numerator and the kg at the denominator 1 lb. 0.4536 kg. , Ib. 0.4536 kg Multiply the original quantity by this conversion ratio. 2.3kg x Ib. = 5.07 Ib. 0.4536 kg 1. 1,357 mm = _____________m 2. 8 L = __________mL 3. 12O kg = __________ g 4. 6,000 s =________ min 5. 24.5 m =_________cm 6. 5 m =__________cm 7. ________ m= 6 km 8. A liter of chloroform has a mass of 1490 grams. Convert this mass to kilograms. A weightlifter can lift 495 lbs. How many kg is that ? A certain car has mass of 1920 kg . How many tons is that ? 55 km to m hr s