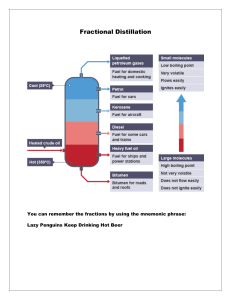
Separation Techniques: Objectives: - describe techniques for the separation of mixtures, including simple distillation, fractional distillation, filtration, crystallisation and paper chromatography. Outcomes: • Be able to describe & explain distillation & paper chromatography • Be able to carry out chromatography to separate dyes Distillation: • Distillation is a process that can be used to obtain a pure liquid from another liquid or a solid. It works when the substances have different boiling points. • This is the sequence of events in distillation: heating → evaporating → cooling → condensing Fractional distillation • Remember: Distillation is a process that can be used to collect one pure liquid from a mixture of substances. • Fractional distillation takes advantage of the fact that different liquids will have all different boiling points and allows you to collect multiple pure liquids • heating → evaporating → cooling → condensing Liebig E.G: How does fractional distillation work? 6 of 37 © Boardworks Ltd 2006 Fractional distillation of air Colder at the top 1. Air has to be condensed into a liquid. This happens at 200oC. 2. This is done by compressing or squashing the air and the use of cold water. 3. At -200oC carbon dioxide and water are solids so can be easily taken out. Warmer at the bottom 4. Nitrogen boils at -196oC so it can be removed from the top of the column as a gas. 5. At -185oC oxygen is still a liquid so can be taken out the bottom of the column. • • • • • • • • • Twig videos Filtration - https://www.twig-world.com/experiment/chemical-filtration-4131/ Evaporation - https://www.twig-world.com/experiment/filtration-and-evaporation-4130/ Salt separating mixtures - https://www.twig-world.com/film/salt-separating-mixtures-1476/ Fractional distillation - https://www.twig-world.com/film/fractional-distillation-1371/ Distillation of ink - https://www.twig-world.com/experiment/distillation-of-ink-4129/ Chromatography - https://www.twig-world.com/search/?search=chromatography&search-btn= Sublimation - https://www.twig-world.com/experiment/fire-extinguisher-sublimation-4128/ Centrifuge - https://www.youtube.com/watch?v=KEXWd3_fM94 Separation techniques: Objectives: - describe techniques for the separation of mixtures Objectives: D: Be able to identify simple separation techniques A-C: - Describe filtration and crystallisation and their uses Filtration & crystallisation: • Filtration: separates a solid from a liquid • Crystallisation: if a solid is dissolved in water it can be recovered by evaporation or crystallisation • When a solution is heated, the liquid will start to evaporate. • When the solution becomes saturated, the solute will begin to crystallise and will appear in the solution • So how would we carry out a practical to separate sand, salt and water? When you are done: • Write a paragraph to describe this experiment. Make sure all of the following words are used: dissolve, solvent, filter, filtrate, evaporate, solute, crystallise, crystal.