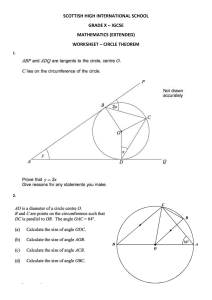
IGCSE CHEMISTRY LABORATORY MANUAL GHIYASUDD IN INTERNATIO NAL S C H O O L GIS MANUAL IGCSE CHEMISTRY (0620) LAB 0 IGCSE CHEMISTRY LABORATORY MANUAL T A BL E O F C O N TE N T I. INTRODUCTION II. PROPERTIES OF MATTER AND CRITERIA OF PURITY 1. DETERMINATION OF MELTING POINT 2. DETERMINATION OF BOILING POINT 3. FACTORS AFFECTING DIFFUSION – DIFFUSION OF AMMONIA AND HYDROGEN CHLORIDE III. METHODS OF SEPARATION AND PURIFICATION 4. PREPARATION OF COPPER SULPHATE BY CRYSTALLISATION METHOD 5. FILTRATION 6. SUBLIMATION 7. CHROMATOGRAPHY 8. FRACTIONAL DISTILLATION OF ETHANOL AND WATER MIXTURE IV. TEST FOR IONS 9. QUALITATIVE ANALYSIS OF SIMPLE SALT NO:1 10. QUALITATIVE ANALYSIS OF SIMPLE SALT NO:2 11. QUALITATIVE ANALYSIS OF SIMPLE SALT NO:3 12. QUALITATIVE ANALYSIS OF SIMPLE SALT NO:4 13. QUALITATIVE ANALYSIS OF SIMPLE SALT NO:5 V. QUANTITATIVE CHEMISTRY 14. ACID- ALKALI TITRATION 15. PERMANGANOMETRY 16. ACID- ALKALI TITRATION VI. ELECTROLYSIS 17. ELECTROLYSIS OF MOLTEN SODIUM CHLORIDE 18. PURIFICATION OF COPPER BY ELECTROLYSIS VII. ACID, BASE AND SALT H 19. P OF SOME COMMON SUBSTANCES 20. PREPARATION OF WATER SOLUBLE SALT 21. PREPARATION OF WATER SOLUBLE SALT 22. PREPARATION OF WATER SOLUBLE SALT BY TITRATION VIII. RATE OF REACTION 23. EFFECT OF TEMPERTURE ON RATE OF REACTION 24. EFFECT OF A CATALYST ON RATE OF REACTION 25. EFFECT OF CONCENTRATION ON RATE OF REACTION 26. EFFECT OF SURFACE AREA ON RATE OF REACTION IX. ORGANIC CHEMISTRY 27. FERMENTATION OF GLUCOSE 28. OXIDATION OF ALCOHOL 29. REACTION OF METALS WITH ALCOHOL X. ENERGETICS OF REACTION 30. DETERMINATION OF HEAT OF COMBUSTION OF ETHANOL XI. METALS 31. DISPLACEMENT REACTION OF METALS 32. COMPARISON OF METALS REACTIVITY GIS MANUAL IGCSE CHEMISTRY (0620) LAB 1 IGCSE CHEMISTRY LABORATORY MANUAL I. INTRODUCTION CHEMISTRY LABORATORY A Chemistry laboratory is a workshop for chemists. Here students learn the technique of the preparation, identification and estimation of chemical substances. Before starting experiment a student must know from where to get the apparatus required for the given experiment and the placement of the chemicals to be used. A student should know the proper use of each equipment and the precautions to be observed while working in the laboratory. A chemistry laboratory is provided with the following fittings with which the student must become familiar. 1. Demonstration Table Before starting the experiment, the teacher gives instructions and demonstrates the concerned experiment on demonstration table. In chemistry laboratory, no seats are given to students, so students stand around demonstration table and note the instruction from the teacher. 2. Students working table A number of wooden or concrete tables are given for working. Each seat is provided with: (a) Reagent shelves. Reagents or chemicals to be used are placed on the reagent shelf. These are the reagents which are commonly used such as dilute acids and bases. (b) Sinks and water taps (c) Gas taps 3. Side shelves Mostly there are two big shelves fitted on the walls of the laboratory. Reagents and chemicals, which are less frequently used, are placed in these shelves. Sometimes solid chemicals are placed in a separate shelf. 4. Fume Cup- board There is at least one fume cupboard in the corner of the laboratory. All experiments giving out poisonous gases or vapours are performed in this cup- board 5. Balance room It is a small room attached to each laboratory. Here a number of balances are kept for weighing the substances. 6. Exhaust fans Two exhaust fans are provided at two corners of the laboratory for the removal of the poisonous gases and vapours from the laboratory. COMMON LABORATORY APPARATUS The apparatus which are commonly used by the students of secondary classes is described below. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 2 IGCSE CHEMISTRY LABORATORY MANUAL 1. Beakers Beakers of different sizes such as 150 ml, 200 ml made of soft glasses or corning glass. Beakers are used for taking various liquids. 2. Test tubes Test tubes of different sizes are available. Small test tubes used for salt analysis known as centrifuging tube and boiling tubes are also available. 3. Conical flasks It is used in titration 4. Funnel It is used for filtration or for pouring solutions. 5. Measuring flask It is used in quantitative analysis when we have to prepare a solution with a particular volume. There are flasks of 50 ml, 100 ml and 250 ml capacity. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 3 IGCSE CHEMISTRY LABORATORY MANUAL 6. Glass- rod. It is used for stirring purposes. It is also used as an aid for transferring the liquid in to the funnel. 7. China dish. It is a small vessel made of porcelain. It is used in crystallisation for concentrating a solution. 8. Wire gauze It is placed above the flame of the burner so that the glass vessel being heated does not touch the flame directly and hence is prevented from breaking. 9. Tripod stand It is used for supporting a china dish or a beaker so that it can be heated from below. 10. Burette It is used for titration. In burette liquids can be measured with an accuracy 3 of 0.1cm GIS IGCSE CHEMISTRY (0620) LAB MANUAL 4 IGCSE CHEMISTRY LABORATORY MANUAL 11. Pipette It is used in titration to measure exact quantity of a liquid. Other apparatus with which a student must familiarize are test tube holder, test tube brush, crucible tongs, spatula, Bunsen burner tongs Spatula GIS MANUAL IGCSE CHEMISTRY (0620) Test tube rack LAB 5 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 1 –DETERMINATION OF MELTING POINT AIM The aim of this experiment is to determine the melting point of the given solid substance. THEORY The melting point of a substance may be defined as the temperature at which the substance changes from the solid state to the liquid state. It is a very useful physical constant because a pure substance melts at a definite temperature and has a sharp melting point while an impure substance has a lower melting point and melts over a wide range. Therefore, determination of melting point is a very convenient method to check the purity of a solid substance. More over melting point determination can be used to identify a substance by comparing its melting point with the melting point of known substance. REQUIREMENTS 50 ml beaker, thermometer, iron stand, clamp, tripod stand, stirrer, thin walled capillary tube 8 to 10 cm long and 1 to 2mm diameter, spatula, liquid paraffin. PROCEDURE 1. Powder the crystalline substance. Take a capillary tube and seal its one end by heating. For filling the substances make a heap of the powdered substances on the porous plate. Push the open end of the capillary tube in to the heap. Some substance will enter in to it. Now tap the sealed end of the capillary tube on the porous plate gently. Fill the capillary tube up to 2 – 3 mm. 2. Attach the capillary tube to athermometer which is immersed in a bath of liquid paraffin. The surface tension of the bath liquid is sufficient to hold the capillary tube in position. 3. Heat the beaker slowly and go on stirring the liquid in the beaker so that the temperature remains uniform throughout. For this, a glass loop stirrer is moved up and down. When the 0 temperature is within 15 C of the melting point of the pure substance, flame is lowered. Now the temperature is allowed rising slowly. 4. The temperature is noted when the substance starts melting. The temperature is noted again when it is completely melted. The average of the two readings gives the correct melting point of the substance. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 6 IGCSE CHEMISTRY LABORATORY MANUAL OBSERVATIONS 0 The temperature at which the unknown substance begins to melt, t1 C = ………………………………………………………… 0 The temperature at which the substance completely melts, t2 C = …………………………………………………………. 0 The melting point of the unknown substance = [ ( t1+ t2 )/2] C = …………………………………………………………. DISCUSSIONS 1. How is the determination of the melting point useful? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 2. How does the determination of the melting point help us know about the purity of the compound? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 3. What is sharp melting point? ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 7 IGCSE CHEMISTRY LABORATORY MANUAL ………………………………………………………………………………………………… 4. What is the effect of impurities on the melting point of solids? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 5. Why different solids have different melting points? ………………………………………………………………………………………………… ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 8 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 2 –DETERMINATION OF BOILING POINT AIM The aim of this experiment is to determine the boiling point of the given liquid. THEORY The boiling point of a liquid may be defined as the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure excreted up on the liquid surface. The boiling point of the liquid depends up on the pressureexcreted on the liquid surface. Since the atmospheric pressure is different at different place, therefore a liquid has different boiling points at different places. For the sake of comparison we use the normal boiling points. The normal boiling point of a liquid may be defined as the temperature at which the vapour pressure of the liquid is equal to tone standard atmospheric pressure (760 mm). REQUIRMENTS 100 ml corning glass beaker, a small thin walled test tube, thermometer, a capillary tube, a tripod stand, wire gauze, stirrer, iron stand and clamp, liquid paraffin or concentrated sulphuric acid and the given liquid. PROCEDURE 1. Take a small test tube and fill it two third with the given liquid whose boiling point is to be determined. Fix this tube to the thermometer with a rubber band. The rubber band should be fixed near the mouth of the test tubeso that it remains outside the liquid paraffin bath. Adjust the liquid so that the bottom of the liquid is somewhere at the middle of the thermometer bulb. 2. Clamp the thermometer carrying test tube in an iron stand through a cork. Lower the thermometer along with the tube in to aliquid paraffin bath. Adjust the thermometer so that its bulb is well under the acid and open end of the tube with the rubber band is sufficiently outside the acid bath. 3. Take a capillary tube 5 – 6 cm in length and seal it at about 1 cm from one end by heating it in flame and giving a slight twist. Place this capillary in the test tube so that sealed part of it stands in the liquid. 4. Start heating the liquid paraffin bath slowly and stir the bath gently. At first a bubble or two will be seen escaping at the end of the capillary dipping in the liquid, but soon a rapid and continuous stream of air bubbles escapes from it. This is the stage where the vapour pressure of the liquid in the sealed capillary just exceeds the atmospheric pressure. Note the temperature when continuous stream of bubbles starts coming out. Remove the flame and note the temperature when the evolution of bubbles from the end of the capillary tube just stops. The mean of the two temperatures gives the boiling point of the liquid. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 9 IGCSE CHEMISTRY LABORATORY MANUAL 0 5. Allow the temperature fall by 10 C and repeat the heating and again note the boiling point. OBSERVATIONS 0 Boiling point, t1 C = …………………………………………………………………… 0 Boiling point, t2 C = …………………………………………………………………… 0 Mean [ ( t1+ t2 )/2] C = ………………………………………………………………… DISCUSSIONS 1.Define boiling point? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 2. What is the effect of increase of pressure on the boiling point? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 3. What is the effect of impurities on the boiling point of liqlids? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 4. What is the effect of decrease of pressure on the boiling point? ………………………………………………………………………………………………… 5. Why is food cooked more quickly in a pressure cooker? ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 10 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 3 –DIFFUSION OF AMMONIA AND HYDROGEN CHLORIDE GAS AIM To compare the rate of diffusion of ammonia gas with that of hydrogen chloride. THEORY Diffusion refers to the process by which molecules intermingle as a result of their kinetic energy of random motion. Consider two containers of gas A and B separated by a partition. The molecules of both gases are in constant motion and make numerous collisions with the partition. If the partition is removed as in the lower illustration, the gases will mix because of the random velocities of their molecules. In time a uniform mixture of A and B molecules will be produced in the container. The tendency toward diffusion is very strong even at room temperature because of the high molecular velocities associated with the thermal energyof the particles. PROCEDURE Cotton wool soaked in concentratedammonia solution, NH3(aq)and concentrated hydrogen chloridesolution (also called hydrochloric acid) HCl(aq)are placed at each end of a sealed tube. The cotton wool with ammonia solution gives off ammonia molecules (NH3). The cotton wool with hydrochloric acid gives off hydrogen chloride molecules (HCl). HCl and NH3 molecules diffuse through the air towards each other. When they meet, they react to form a white powder called ammonium chloride, NH4Cl. hydrogen chloride HCl(g) The + ammonia + NH3(g) ammonium chloride. NH4Cl(s) sign shows that the reaction is reversible. Note that lighter (smaller) particles move more quicklythan heavier (larger) ones at the same temperature. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 11 IGCSE CHEMISTRY LABORATORY MANUAL OBSERVATION The point at which the white spot is formed is …………………………………………………………………. The distance from this point to the cotton bud dipped in HCl = …………………………………………………………………. The distance from this point to the cotton bud dipped in NH3 = …………………………………………………………………. The ring of white powder is closer to ………………………………………………………………………. CONCLUSION The ring of white powder is closer to the HCl than the NH 3.This is because the NH3 molecules are lighter (smaller) and have diffused more quickly through the air in the tube (you can work out which molecule is lighter by looking at the RFM). DISCUSSION 1. Define the term diffusion? ………………………………………………………………………………………… ………………………………………………………………………………………… 2. What are the factors affecting diffusion ………………………………………………………………………………………… ………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 12 IGCSE CHEMISTRY LABORATORY MANUAL 3. Arrange the following gases in the increasing order of their rate of diffusion. Carbon dioxide, Methane, ethane, ammonia, hydrogen sulphide ………………………………………………………………………………………… ………………………………………………………………………………………… 4. Explain why the rate of diffusion is less in liquid than in gas ………………………………………………………………………………………… ………………………………………………………………………………………… 5. Describe how the rate of diffusion increases with temperature ………………………………………………………………………………………… ………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 13 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 4–PREPARATION OF COPPER SULPHATEBY CRYSTALLISATION METHOD AIM The aim of this experiment is to obtain pure dry crystals of copper sulphate by crystallization method. THEORY Copper(II) oxide solid reacts with dilute sulphuric acid to form copper sulphate salt and water by the neutralization method. CuO(s) + H2SO4 (aq) CuSO4(aq) + H2O (l) Copper sulphate can be obtained from the aqueous solution as crystals. Pure copper sulphate always has sharp melting point REQUIREMENTS Solid copper (II) oxide powder, dil. Sulphuric acid, 500 ml beakers, glass rod, watch glass, tripod stand, wire gauge, filter funnel and filter paper, Evaporating dish, Apparatus to check the melting point of copper sulphate PROCEDURE 3 Approximately 10g of copper (II) oxide is added to 50 cm of dilute sulphuric acid taken in a beaker and stir the solution properly for about 2 minutes. Filter off the unreacted copper(II)oxide and the filtrate is an aqueous solution of copper sulphate. Heat the aqueous salt solution in an evaporating dish for about 5 minutes well below its boiling point and pour the sample of concentrated aqueous solution in to a clean dry watch glass. Then leave the hot solution for natural cooling for the formation of crystals. Crystals produced can be obtained by filtration and press them in between the pads of filter paper to make it dry. Purity of the crystals can be checked from the melting point value obtained. Pure substance always has sharp melting point. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 14 IGCSE CHEMISTRY LABORATORY MANUAL OBSERVATION The melting point of the prepared sample of copper sulphate is …………………………………………………………….. The melting point of pure sample of copper sulphate is …………………………………………………………….. CONCLUSION Therefore the produced sample of copper sulphate is ……………………………………………………………….. (Pure/ moderately pure/ impure) DISCUSSIONS 1.What do you mean by melting point of a substance? ………………………………………………………………………………………………… ………………………………………………………………………………………………… 2. What is the colour of hydrated copper (II) sulphate crystals? ………………………………………………………………………………………………… 3. Suggest two other salts which can be obtained by crystallization method? ………………………………………………………………………………………………… ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 15 IGCSE CHEMISTRY LABORATORY MANUAL 4. Determine the formula of hydrated copper (II) sulphate? ………………………………………………………………………………………………… 5. Explain why copper (II) sulphate cannot be produced by crystallization method. ………………………………………………………………………………………………… ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 16 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 5 - FILTRATION AIM OF THE EXPERIMENT: Filtration to separate sand and a soluble salt MATERIALS AND EQUIPMENTS: Filter funnel, paper and stand Glass rod, two beakers, evaporating basin. Tripod, gauze, Bunsen burner Mixture of sand and a soluble salt INSTRUCTION: Pour the sand and salt mixture into the beaker Fill the beaker to about 25cm mark with water Stir the mixture to dissolve the salt Filter the mixture into the evaporating basin ( The filtrate is salt solution; the residue is sand) 3 USEFUL TECHNIQUES: Proper use of filter paper – Make sure that the filter paper is properly folded ( i.e. into halves and then into quarters), so that it makes a cone inside the filter funnel. Wet the inside of the filter paper this keeps the filter paper in position. Pouring liquids – use a glass rod as a guide held over the top of the beaker , the liquid can then be directed into the centre of the filter paper without spilling it. During filtering do not touch the paper with the glass rod – it might tear it. DISCUSSIONS 1. What is the name of the solid particles left in the filter funnel after filtration? ……………………………………………………………………………………… 2. What is the name of the liquid collected after filtration? ……………………………………………………………………………………… 3. Name any one industrial use of filtration? ……………………………………………………………………………………………... GIS IGCSE CHEMISTRY (0620) LAB MANUAL 17 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 6 – SUBLIMATION AIM OF THE EXPERIMENT: Investigating the sublimation of ammonium Chloride MATERIALS EQUIPMENTS: Eye protection glass ( ammonium chloride is harmful and irritating to eye) Test tube Bunsen burner Retort stand or test tube holder Ammonium chloride INSTRUCTION: 1.Put enough ammonium chloride into a test tube to just fill the base.Plug the tube with mineral wool. 2.Warm the tube so that the heat is concentrated at the base of the test tube Observation: ...................................................................................................................................................... ………………………………………………………………………………………………… ………………………………………………………………………………………………… Definition of Sublimation: ………………………………………………………………………………………………… ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 18 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 7 – CHROMATOGRAPHY AIM OF THE EXPERIMENT: Chromatography (Investigation of felt –pen ink) MATERIALS AND EQUIPMENTS: Eye protection glass Square chromatography paper with punched holes Ruler and pencil Selection of felt pens including black Solvent INSTRUCTION: 1.Draw a pencil reference line across the paper about 2cm from the base and parallel with it. 2.In pencil , mark six dots on the line, atleast 1cm apart 3.Put a different ink sample on each dot. 4.Write the colour at the top of the paper opposite to the dots for reference. 5.Pour about 1cm depth of solvent on a container 6.Submerge the chromatography paper in the solvent OBSERVATION: ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 19 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 8 – FRACTIONAL DISTILLATION OF ETHANOL AND WATER MIXTURE AIM To separate a mixture of ethanol and water by fractional distillation method. THEORY Some liquids, such as oil and water, do not mix. It is easy to separate them out into their components. However, some liquids dissolve in one another to form a solution. They are called miscible because they mix together. They are much more difficult to separate. An example of this type of mixture is crude oil.Distillation is the process used for separation. Each of the liquids in the mixture has a different boiling point. If we heat the mixture, the liquid with the lowestboiling point will vaporize first. We can collect the vapor and condense it to form the pure liquid. Then we can raise the temperature to boil off anotherliquid in the mixture. Each liquid collected is called a fraction, and the process iscalled fractional distillation. In this experiment you will be separating a mixtureof two liquids with similar boiling points – ethanol and water. REQUIREMENTS mixture of ethanol and water (about30 ml per experiment),boiling chips ,Erlenmeyer flask with a rubberstopper with a center hole of thecorrect size to fit the delivery tubeof the fractionating column, fractionating column ,–10°C to 110°C thermometer,condenser,tap water, stoppers with holes to connectapparatus,2 small beakers,Bunsen burner,tripod and gauze,spatula, 2 stands and 2 ring clamps,lab jack, marker pen,safety glasses PROCEDURE 1. You will need to work in a pair to set up the equipment for this experiment. 2. Place the mixture provided by your teacher in the Erlenmeyer flask. 3. Add half a spatula of boiling chips to the mixture. 4. Place the flask on top of a tripod and gauze. 5. Place a stopper in the neck of the flask and carefully feed the end of thefractionating column through the stopper. Use a stand and a ring clamp tosecure the fractionating column in place. 6. Place a second stopper with a thermometer fed through it in the top of thefractionating column. Make sure the bulb of the thermometer is level with theside arm of the fractionating column. 7. Attach the condenser to the side tube of the fractionating column. Secure thecondenser in place using a second stand and ring clamp. 8. Make sure the cold tap water is fed into the condenser at the end nearest tothe fractionating column. 9. Place a beaker, marked A, under the end of the condenser to collect thedistillate produced (see diagram). 10. Set up a Bunsen burner under the tripod and gauze, and light it. 11. Heat the mixture to 78°C using a small flame and watch what happens. Record your observations in the data table below. 12. Keep the temperature at 78°C until no more liquid is collected in beaker A. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 20 IGCSE CHEMISTRY LABORATORY MANUAL 13. Remove beaker A and place it well away from the Bunsen burner. 14. Place a second beaker, marked B, under the end of the condenser and heat theflask to 100°C. Collect any liquid that is produced. 15. Switch off all Bunsen burners. OBSERVATION RESULT AND ANALYSIS The mixture you placed in the Erlenmeyer flask was ethanol and water. Theboiling point of ethanol is 78°C and the boiling point of water is 100°C. 1. What did you observe when the temperature reached 78°C? ………………………………………………………………………………………………… 2. What is the name of the liquid collected in beaker A? GIS IGCSE CHEMISTRY (0620) LAB MANUAL 21 IGCSE CHEMISTRY LABORATORY MANUAL ………………………………………………………………………………………………… 3. How did this liquid get into beaker A? Explain the physical changes it wentthrough on its journey from the Erlenmeyer flask to the beaker. ………………………………………………………………………………………………… 4. What did you observe when the temperature reached 100°C? ………………………………………………………………………………………………… 5. What is the name of the liquid collected in beaker B? ………………………………………………………………………………………………… 6. What is the purpose of the fractionating column? ………………………………………………………………………………………………… 7. What tests could you apply to the two liquids to establish their identities? ………………………………………………………………………………………………… 8. Which substance has stronger bonds between its molecules? ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 22 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 9– QUALITATIVE ANALYSISOF SIMPLE SALT NO: 1 Introduction Chemists often have to identify the composition of unknown substances. Thisexperiment involves identifying the cations and anions in various salt solutions. What to record What to do 1. Dissolve the unknown substance in deionised water. 5–10 cm3 of solution may beneeded. 2. Using the analysis table, test small aliquots (portions). 3. Repeat for the other unknown substances. Analysis table Testing salts for anions and cations. For anions: carry out the three tests A,B and C below: GIS IGCSE CHEMISTRY (0620) LAB MANUAL 23 IGCSE CHEMISTRY LABORATORY MANUAL For cations: carry out the two tests D and E below: Flame tests. 1. Slightly open the air hole of the Bunsen burner. 2. Heat a piece of nichrome wire in a Bunsen flame until the flame is no longercoloured. 3. Dip the loop at the end of the wire into some water. 4. Dip the loop into an unknown salt. 5. Hold the wire in the edge of the flame. 6. Record the colour and identify the cation using the table below. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 24 IGCSE CHEMISTRY LABORATORY MANUAL Safety Wear eye protection. Some of the unknowns may be toxic or corrosive. Conclusion The given inorganic salt contains the Anion: …………………………………………. Cation: ………………………………………… Formula of the given compound is: ……………………………………………………….. Discussions 1. Write word and ionic equations for those reactions that give a positive result. ………………………………………………………………………………………………… ………………………………………………………………………………………………… ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 25 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 10 – QUALITATIVE ANALYSISOF SIMPLE SALT NO: 2 Topic Qualitative analysis. Timing 1–2 hours. Description Students attempt to identify the anions and cations present in a salt by a combinationof tests. Apparatus and equipment (per group) Test-tubes, test tube holder and test tube stand, Test tube bush Chemicals (per group) Access to: Full range indicator paper –3 Ammonia solution 2 mol dm –3 Sodium hydroxide solution 0.4 mol dm (Irritant) –3 Hydrochloric acid solution 0.4 mol dm –3 Barium chloride solution 0.1 mol dm (Harmful) –3 Limewater solution 0.02 mol dm –3 Nitric acid 0.4 mol dm (Irritant) –3 Silver nitrate solution 0.1 mol dm Unknown substances labelled A, B, C …each might contain one of the following anions and one of the following cations: – 2– 2– – – – Anions - OH , SO4 , CO3 , Cl , Br ,I , NO3 Cations - H , Ca , Cu , Fe , Fe , NH4 + 2+ 2+ 3+ 2+ – + Extension – for flame tests Nichrome wire loops attached to wooden handles (cleaned before lesson in Concentrated hydrochloric acid). Procedure Conduct qualitative test for the anions and cations listed using the analysis able. Safety Wear eye protection. Ammonia solution causes burns and gives off ammonia vapour which irritates the eyes, lungs and respiratory system. Sodium hydroxide can cause burns and is dangerous to the eyes. Hydrochloric acid can cause burns. Barium chloride is harmful by inhalation and if swallowed. Nitric acid causes burns. Silver nitrate solution causes burns. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 26 IGCSE CHEMISTRY LABORATORY MANUAL Flame tests It is probably inadvisable to use concentrated hydrochloric acid to produce volatile chlorides at this level. This procedure should be effective as long as sodium, which produces a persistent yellow colour, is not given as an unknown. Background theory A knowledge of precipitation reactions is helpful as is pre-knowledge of the chemistry of the tests. Otherwise, the students should test known substances to ensure they know what a positive result is. Conclusion The given inorganic salt contains the Anion: …………………………………………. Cation: ………………………………………… Formula of the given compound is: ……………………………………………………….. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 27 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 11 – QUALITATIVE ANALYSISOF SIMPLE SALT NO: 3 AIM To analyse the following salt for the identification and conformation of anion and cations present. THEORY Ionic salts are formed from an anion and a cation Presence of anion and cation can be identified with the help of analysis table. APPARAUS REQUIRED Test-tubes, test tube holder and test tube stand, Test tube bush,three aqueous solutions P, Q, and R. PROCEDURE AND OBSERVATIONS All studentsare given with three aqueous salt solutions P, Q, and R.Complete the test and observations which lead to the conclusions. CONCLUSION The anions present in P, Q and R respectively are …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 28 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 12 – QUALITATIVE ANALYSISOF SIMPLE SALT NO: 4 AIM To analyse the following salt for the identification and conformation of anion and cations present. THEORY Ionic salts are formed from an anion and a cation Presence of anion and cation can be identified with the help of analysis table. APPARAUS REQUIRED Test-tubes, test tube holder and test tube stand, Test tube bush, substance T PROCEDURE The following tests are conducted on the substance T as per the following table. Note the observation in the spaces provided. Complete the table by describing these observations and suggest the test and observations which lead to the conclusion from test 4 Conclusion The formula of the given substance T is ……………………………………….. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 29 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 13 – QUALITATIVE ANALYSISOF SIMPLE SALT NO: 5 AIM To analyse the following salt for the identification and conformation of anion and cations present. THEORY Ionic salts are formed from an anion and a cation Presence of anion and cation can be identified with the help of analysis table. APPARAUS REQUIRED Test-tubes, test tube holder and test tube stand, Test tube bush, Given salt in a watch glass (a mixture of two calcium compounds C and D) PROCEDURE Tests are conducted on the salts C and D according to the test table for anions and cations using the appropriate reagents listed and record your observations in the spaces provided in the observation table. OBSERVATION AND CONCLUSION Complete the observations in the table. (b) Name the gas given off in (a) (iii). …………………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 30 IGCSE CHEMISTRY LABORATORY MANUAL (c) Suggest an explanation for the observation in (a) (v). ………………………………………………………………………………………………… (d) What conclusions can you draw about the identity of the anions in solid C and D? ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 31 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 14 – ACID- ALKALI TITRATION AIM To determine the value of X in the formula of an acid is written as HxA. THEORY The reaction between an acid and alkali to form salt and water is called neutralization + reaction. Neutralisation is the basis of acid alkali titration. In neutralization H ions of the acid is neutralized by the OH of the alkli to form water APPARATUS AND REAGENTS REQUIRED Given solutions S and T, burette, pipette, conical flask, burette stand, clamp, glass rod, white tile, indicator,beaker PROCEDURE S is a 0.0450 mol/dm3 aqueous solution of acid HxA. T is 0.120 mol/dm3 sodium hydroxide. A burette was filled with S. A 25.0 cm3 portion of T was measured into a conical flask. A few drops of methyl orange indicator were added The solution taken in the flask is titrated against the given acid in the burette. Calculations and Observation Summary. Tick the best titration results. Using these results, the average volume of solution S was 3 .................. cm . (d) T is 0.120 mol/dm3 sodium hydroxide. Calculate how many moles of sodium hydroxide are present in 25.0 cm3 of solution T. ................. moles 3 (e) S is 0.0450 mol/dm HxA. Calculate how many moles of acid HxAare present in your average volume of S. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 32 IGCSE CHEMISTRY LABORATORY MANUAL …............... moles (f) Using your answers to (d) and (e) deduce how many moles of sodium hydroxide react with one mole of HxA. ....................moles (g) Using your answer to (f) deduce the value of x in the formula HxA. ......................................... (h) Using your answers to (f) and (g) write an equation for the reaction between sodium hydroxide and HxA. ................................................................................................................................................... Result The value of X in the formula HxA is = GIS IGCSE CHEMISTRY (0620) LAB MANUAL 33 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 15– PERMANGANOMETRY AIM To determine the value of X in the formula of iron (II) sulphate crystals, given as FeSO4.xH2Oand calculate the mass of iron (II) sulphate crystals used in the experiment. THEORY Determination of the amount or the concentration of the salt by titrating against standard potassium permanganate is called permanganometry. 2+ Fe ions in ferrous sulphate salt are oxidized by permanganate solution and there by the permanganate solution undergo reduction. This is an example for a redox reaction and redox titration. APPARATUS AND REAGENTS REQUIRED Given solutions G, iron (II) sulphate crystals, dilute sulphuric acid, burette, pipette, conical flask, burette stand, clamp, glass rod, white tile, and beaker. PROCEDURE (a) A sample of iron(II) sulphate crystals is added to a previously weighed container,which is then reweighed. . Mass of container + crystals = 12.38 g Mass of empty container = 5.42 g Mass of iron(II) sulphate crystals = ……………… g (b) The sample is dissolved in 100 cm3 of dilute sulphuric acid and the solution is made up to 250 cm3 with distilled water. This was solution H.A 25.0 cm3 sample of H was measured into a titration flask. Solution G is run from a burette into the flask containing H until an end-point is reached. Potassium manganate(VII) is purple. The appearance of permanent pink colour indicates the end point. (c) Repeat the titration three times to get close titration values. Summary. Tick (✔) the best titration results. Using these results, the average volume of G was GIS IGCSE CHEMISTRY (0620) LAB MANUAL 34 IGCSE CHEMISTRY LABORATORY MANUAL 3 (a) G is 0.0200 mol/dm3 potassium manganate (VII). .................. cm . Calculate how many moles of KMnO4 were present in the titrated volume of Gcalculated in (c). ..................moles (b) Five moles of FeSO4 react with one mole of KMnO4. Calculate how many moles of FeSO4were present in 25.0 cm3 of H. .................. moles (c) Calculate how many moles of FeSO4 were present in the 250 cm3 of H. .................. moles (d) Using your answers to (c), calculate the mass of FeSO4 in the original sample of FeSO4.xH2O. [Mr: FeSO4, 152.] .................. g GIS IGCSE CHEMISTRY (0620) LAB MANUAL 35 IGCSE CHEMISTRY LABORATORY MANUAL (e) Using your answer to (a) and (d) calculate the mass of water in the sample ofFeSO4.xH2O. .................. g (f) Using our answer to (e) calculate the number of moles of water in the sample ofFeSO 4.xH2O. [Ar:H,1; O, 16] .................. moles (g) Using your answers to (f) and (i) calculate the value of x in FeSO4.xH2O. ................................................................................................................................................... RESULT The value of X in the formula FeSO4.xH2O is ………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 36 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 16 – ACID ALKALI TITRATION AIM OF THE EXPERIMENT To determine the average volume of the alkali required to react completely with the measured amount of the acid taken in a conical flask. INSTRUCTION: 3 Pipette out 25cm of 1M hydrochloric acid into a conical flask. Add 2 drops of phenolphthalein indicator into the flask and swirl Slowly release aqueous sodium hydroxide of know concentration into the flask from the burette and swirl the flask constantly to mix the content. When the pink colour first appear in the flask, stop the addition of sodium hydroxide and note the volume of the alkali used. Repeat the titration steps, adding the noted volume of the alkali into the flask 3 of 25cm of 1M acid. Use the results to complete the following table Summary Tick (_) the best titration results. 3 Using these results the average volume of alkali used was ........................................... cm . GIS IGCSE CHEMISTRY (0620) LAB MANUAL 37 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 17– ELECROLYSIS OF MOLTEN SODIUM CHLORIDE AIM OF THE EXPERIMENT: To verify that the electrolysis of a molten binary salt gives metal deposit at the cathode and the formation of nonmetallic element at the anode. THEORY When electricity passes through molten compounds, like sodium chloride, the ionsmove towards the electrode of opposite charge. Sodium chloride gives sodium metaland chlorine gas. This experiment illustrates what happens when molten sodium chloride is electrolyzed. PROCEDURE Molten sodium chloride is taken in a beaker and which is connected to a DC power supply through two inert graphite electrodes as shown in the diagram below. Pass the electricity through the molten sample and take out the cathode to verify that the metal is deposited at the cathode. Cathode is weighed before and after the experiment to show an increase in weight due to the metal deposit. Collect the chlorine gas in the test tube conform the gas by the test using a damp litmus paper. Observations and calculations Weight of the carbon anode before the experiment = ……………………………………… Weight of the carbon anode after the experiment = ……………………………………… Weight of the metallic sodium deposited = ……………………………………… GISIGCSE CHEMISTRY (0620) LAB MANUAL 38 IGCSE CHEMISTRY LABORATORY MANUAL Discussions 1. What type of element is formed at the negative electrode? …………………………………………………………………………………………………………… 2. What type of element is formed at the positive electrode? …………………………………………………………………………………………………………… 3. Write a general rule for the products formed at (a) the cathode …………………………………………………………………………………………………………… (b) the anode. …………………………………………………………………………………………………………… 4. Write ionic equations for the products formed at (a) the cathode …………………………………………………………………………………………………………… (b) the anode. …………………………………………………………………………………………………………… 4412 Classic GIS IGCSE CHEMISTRY (0620) LAB MANUAL 39 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 18– PURIFICATION OF COPPER BY ELECTROLYSIS EXPECTED LEARNING OUTCOME: Purifying copper by electrolysis. MATERIALS AND EQUIPMENTS: Eye protection Beaker Carbon rod Piece of copper foil 2 cables with crocodile clips at both ends Power pack,6-8V Cooper (II) Sulfate solution SAFETY: Copper(II) sulfate solution is harmful if swallowed and may be irritating to the skin. Do not touch the power pack with wet hands Wear eye protection when carrying out electrolysis INSTRUCTION: Pour about 2cm depth of the copper (II) sulfate solution into the beaker Put in a carbon rod as the electrode and a piece of copper foil as the anode and connect, via crocodile clips and cables ,to a power pack Pass current at a voltage 6 to 8 volts After about 3 minutes , switch off and remove the carbon rod; it should be coated with copper If you replace the cathode , reconnect and pass current for about 15 minutes , eventually the anode will dissolve away. OBSERVATIONS: …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 40 IGCSE CHEMISTRY LABORATORY MANUAL H EXPERIMENT 19 – P OF SOME COMMON SUBSTANCES AIM OF THE EXPERIMENT: TEST THE ACIDITY AND ALKALINITY OF SOME COMMON CHEMICALS AND HOUSHOLD ITEMS. MATERIALS AND EQUIPMENTS: TEST TUBES/ TEST TUBE RACK LITMUS PAPER PHENOLPHTHALEIN HYDROCHLORIC ACID SODIUM HYDROXIDE TOOTH PASTE DETERGENTS VINEGAR LEMON TOMATO INSTRUCTIONS: Take a sample of the labeled substances to a test tube Test the substance with different indicators Record the observation on the table given. Substances Acidic alkaline neutral Toothpaste Tomato Lemon Vinegar Detergent Hydrochloric acid Sodium hydroxide GIS IGCSE CHEMISTRY (0620) LAB MANUAL 41 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 20– PREPARATION OF WATER SOLUBLE SALT AIM OF THE EXPERIMENT: PART 1 (PREPARATION OF CALCIUM CHLORIDE CRYSTALS) INSTRUCTIONS: 3 Take about 15cm of dilute hydrochloric acid into a beaker Add excess of calcium carbonate to the acid and allow it to react so that all the acid is used up. Filter the solid using filter paper and a funnel Transfer the solution into an evaporating dish and warm it slowly until the solution gets concentrated. Stop warming and allow the saturated solution to cool to let the crystals form Filter off the crystals. Wash them with little distilled water. Dry the crystals between filter papers. Observation: …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… Discussion: GIS White coloured calcium carbonate reactswithdilute hydrochloric acid to form calcium chloride. Chemical equation; IGCSE CHEMISTRY (0620) LAB MANUAL 42 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 21– PREPARATION OF WATER SOLUBLE SALTS AIM OF THE EXPERIMENT: PART 2: PREPARATION OF COPPER (II) SULFATE INSTRUCTIONS: 3 Take about 15cm of dilute sulfuric acid into a beaker Add excess of copper oxide to the acid and allow it to react so that all the acid is used up. Filter the solid using filter paper and a funnel Transfer the solution into an evaporating dish and warm it slowly until the solution gets concentrated. Stop warming and allow the saturated solution to cool to let the crystals form Filter off the crystals. Wash them with little distilled water. Dry the crystals between filter papers. OBSERVATION: …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… DISCUSSION: Black coloured copper(II) oxide reacts with dilute sulfuric acid to form copper (II) sulfate. CHEMICAL EQUATION: …………………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 43 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 22– PREPARATION OF WATER SOLUBLE SALTS BY TITRATION AIM OF THE EXPERIMENT: PART 3 (PREPARATION OF POTASSIUM CHLORIDE) TITRATION METHOD. Instructions: GIS 3 Pipette out 25cm of 1M hydrochloric acid into a conical flask. Add 2 drops of phenolphthalein indicator into the flask and swirl Slowly release aqueous sodium hydroxide of know concentration into the flask from the burette and swirl the flask constantly to mix the content. When the pink colour first appears in the flask, stop the addition of sodium hydroxide and note the volume of the alkali used. Repeat the titration steps, this time without the indicator, adding the noted 3 volume of the alkali into the flask of 25cm of 1M acid. Heat the solution so that the water will evaporate and the solution becomes saturated. Now cool to let the crystals form. Filter the crystals and dry between filter paper. IGCSE CHEMISTRY (0620) LAB MANUAL 44 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 23 – EFFECT OF TEMPERATURE ON RATE OF REACTION AIM OF THE EXPERIMENT: To investigate the effect of temperature on the rate of reaction CHEMICALS NEEDED Sodium thiosulfate Hydrochloric acid APPARATUS NEEDED Conical flasks Marker (Black) White paper Bunsen burner Thermometer Stop watch Water bath (20 ˚C, 30 ˚C, 40 ˚C, 50 ˚C, 60 ˚C) METHOD 3 1. 2. 3. 4. 5. Measure about 25cm of sodium thiosulfate into a conical flask. Draw a cross on a white sheet of paper provided to you. Place the conical flask on top of the cross. Find out the temperature of the solution using a thermometer. 3 Add about 10 cm of dilute hydrochloric acid into the conical flask. Start the timer at the same time. 6. Observe the cross from the top of the flask and record the time taken for the cross to disappear. 7. Repeat the same procedure with sodium thiosulfate at different temperatures using the water bath provided. 8. Record the temperature and the time taken for the cross to disappear. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 45 IGCSE CHEMISTRY LABORATORY MANUAL RESULTS: TEMPERATURE / ⁰C TIME TAKEN FOR THE CROSS TO DISAPPEAR/ SECONDS Plot the graph of temperature (y-axis) against time (x-axis) on the grid provided and find out the rate of this reaction at 24⁰C and 45⁰C. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 46 IGCSE CHEMISTRY LABORATORY MANUAL CONCLUSION ------------------------------------------------------------------------------------------------------------------------- GIS IGCSE CHEMISTRY (0620) LAB MANUAL 47 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 24 – EFFECT OF A CATALYST ON RATE OF REACTION AIM OF THE EXPERIMENT: To investigate the effect of a catalyst on the rate of reaction MATERIALS AND EQUIPMENTS Hydrogen peroxide solution Manganese (IV) oxide powder – as a catalyst Conical flasks Stop watch Gas syringe (with stand) METHOD 1. 2. 3. 4. Set up the apparatus as shown below. Pour a known volume of hydrogen peroxide into a conical flask and allow it to stand. 3 Record the time taken to collect 100 cm of oxygen. Repeat the same procedure. This time add a small known amount of solid manganese (IV) oxide to the hydrogen peroxide solution. 3 5. Record the time taken to collect 100 cm of oxygen 2 H2O2 → 2 H2O + O2 GIS IGCSE CHEMISTRY (0620) LAB MANUAL 48 IGCSE CHEMISTRY LABORATORY MANUAL RESULTS Hydrogen peroxide status Hydrogen peroxide alone Time taken to collect 100 cm3 of oxygen Hydrogen peroxide with a small amount of MnO2 Hydrogen peroxide with a large amount of MnO2 CONCLUSION GIS IGCSE CHEMISTRY (0620) LAB MANUAL 49 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 25 – EFFECT OF CONCENTRATION ON RATE OF REACTION AIM OF THE EXPERIMENT: To investigate the effect of concentration on rate of reaction CHEMICALS NEEDED Hydrochloric acid of different concentrations Equal mass of magnesium ribbons APPARATUS NEEDED Conical flasks Marker Stop watch Graduated gas syringe METHOD 1. 2. 3. 4. 3 Add 20 cm of hydrochloric acid into a conical flask. Put a piece of magnesium ribbon into the flask and start the timer simultaneously. Measure the time taken to complete the reaction. Record the results in the table. RESULTS Acid concentration (mol/dm3) Time taken to complete the reaction (sec) CONCLUSION ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------Note: we can also measure the specific volume of hydrogen gas liberated in unit time in this reaction by using a gas syringe and a stop watch and hence determine the rate of reaction. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 50 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 26 – EFFECT OF SURFACE AREA ON RATE OF REACTION AIM OF THE EXPERIMENT: TO INVESTIGATE THE EFFECT OF SURFACE AREA ON THE RATE OF REACTION MATERIALS AND EQUIPMENTS: 3 Hydrochloric acid (2 mol/dm ) Calcium carbonate (Lumps, Crystals, Fine powder – 2 grams) Conical flasks Stop watch Gas syringe (with stand) METHOD 3 1. Place 20cm of hydrochloric acid in a conical flask. 2. Set up the apparatus shown below. 3. Take 2g of calcium carbonate lumps. Add the pieces of calcium carbonate to the flask and quickly insert the rubber bung. Start the timing at the same time. 3 4. Measure the time taken to collect 100cm of carbon dioxide gas produced in the reaction. 5. Record the results in the table below. 6. Repeat the same procedure for calcium carbonate crystals and powder. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 51 IGCSE CHEMISTRY LABORATORY MANUAL RESULTS Size of calcium carbonate Lumps Time taken to collect 100cm3 of carbon dioxide/sec Crystals Powder CONCLUSION --------------------------------------------------------------------------------------------------------------------------- ................................................................................................................................................................. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 52 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 27 – FERMENTATION OF GLUCOSE AIM OF THE EXPERIMENT: To prepare a sample of ethanol by fermenting glucose MATERIALS AND EQUIPMENTS. Conical flask Measuring Cylinder Yeast Stopper with delivery tube Spatula Lime water Large beaker or basin Warm water at about 37° C Boiling tube Glucose solution INSTRUCTIONS: 3 Set up the apparatus as shown in the diagram below. Place about 30 cm of the glucose solution in the conical flask. Then add 2 spatula measures of yeast. Immerse the conical flask in a large beaker or basin of water at 37° C. When fermentation begins, remove the beaker of warm water. Leave the set up in a warm place for fermentation process to go to completion. Observe any changes in the limewater and the contents of the conical flask during fermentation. At the end of the experiment, remove the stopper and smell the mixture in the conical flask. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 53 IGCSE CHEMISTRY LABORATORY MANUAL OBSERVATIONS ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- CONCLUSION: DISCUSSION 1. What gas is produced in the fermentation process? Explain how you know. ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 2. Name the product formed in the reaction flask. --------------------------------------------------------------------------------------------------------------------------- 3. Write the equation for the fermentation of glucose. --------------------------------------------------------------------------------------------------------------------------- 4. What would you do to separate the product from the reaction mixture? ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- GIS IGCSE CHEMISTRY (0620) LAB MANUAL 54 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 28 – OXIDATION OF ALCOHOL AIM OF THE EXPERIMENT: To investigate the oxidation property of alcohol MATERIALS AND EQUIPMENTS: Boling tubes Clamp Stopper fitted with delivery tube Bunsen burner Ice water Beaker Ethanol Sulfuric acid Potassium dichromate solution/potassium permanganate solution GIS IGCSE CHEMISTRY (0620) LAB MANUAL 55 IGCSE CHEMISTRY LABORATORY MANUAL INSTRUCTIONS: Place potassium dichromate solution in the boiling tube. Then add a little dilute sulfuric acid to it. Add a little ethanol Set up the apparatus and draw Gently heat the boiling tube. Record any colour change to the solution. OBSERVATION: …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… CONCLUSION: …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… DISCUSSION 1. Suggest the name of the product formed in oxidation of ethanol. ---------------------------------------------------------------------------------------------------------------2. Explain the colour change of the solution. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 56 IGCSE CHEMISTRY LABORATORY MANUAL ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------3. Write down the equation for the reaction. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 57 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 29 – REACTION OF METALS WITH ALCOHOL AIM OF THE EXPERIMENT: To investigate the reaction of alcohol with a metal THEORY: If a small piece of sodium is dropped in to some ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colourless solution of sodium ethoxide, CH 3CH2ONa. MATERIALS AND EQUIPMENTS: Sodium test tube Ethanol match box Wooden splint Instructions: 3 Measure a few cm of ethanol in a test tube and add a piece of sodium. Test for any gas evolved. OBSERVATION: CONCLUSION --------------------------------------------------------------------------------------------------------------------------EQUATION --------------------------------------------------------------------------------------------------------------------------- GIS IGCSE CHEMISTRY (0620) LAB MANUAL 58 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 30– DETERMINATION OF HEATOF COMBUSTION OF ETHANOL AIM OF THE EXPERIMENT: The aim of this experiment is to determine the heat of combustion of ethanol.i.e. the enthalpy change for the reaction: THEORY The enthalpy of combustion of a substance is the energy released when one mole of the substance is completely burned in oxygen. CH3CH2OH (l) + 3O2 (g) 2CO2 (g) + 3H2O (l) The heat energy gained by the water (Eh) can be calculated using the formula: Where c = the specific heat capacity of the water (the heat energy needed to raise the temperature of 1 kg of water by 1 °C) and has the value 4.18 kJ kg-1 °C-1. m = the mass (in kg) of water being heated. (The density of water is 1.00 g cm-3 or 1.00 kg l1) = the rise in temperature in °C. REQUIREMENTS spirit burner (containing ethanol) thermometer copper can measuring cylinder clamp stand and clamp balance draught shield PROCEDURE A measured mass of ethanol is burned in a spirit burner and the heat released is transferred to a copper can containing a known volume of water. From the resulting temperature rise, the enthalpy of combustion of ethanol can be calculated. In this experiment we assume that all the heat released in the combustion reaction is absorbed only by the water in the copper can. step by step procedure is as follows. 1. Weigh the spirit burner (already containing ethanol) with its cap on and record its mass. (The cap should be kept on to cut down the loss of ethanol through evaporation) 2. Using the measuring cylinder, measure out 100 cm3 of water into the copper can. 3. Set up the apparatus as directed by your teacher/lecturer. 4. Measure and record the temperature of the water. 5. Remove the cap from the spirit burner and immediately light the burner. 6. Slowly and continuously stir the water with the thermometer. When the temperature has risen by about 10 °C, recap the spirit burner and measure and record the maximum temperature of the water. 7. Reweigh the spirit burner and record its mass. CALCULATION Initial mass of the spirit burner = ……………….... Final masses of the spirit burner = ………………. The mass of ethanol GIS IGCSE CHEMISTRY (0620) = ………………. LAB MANUAL 59 IGCSE CHEMISTRY LABORATORY MANUAL Initial temperature = ………………… Final temperature = ………………… the rise in temperature = ………………… Mass of water taken(m) = …………………g Enthalpy change of the reaction = GIS IGCSE CHEMISTRY (0620) LAB MANUAL = ………………… kJ/mol 60 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 31– DISPLACEMENTREACTION OF METALS AIM OF THE EXPERIMENT: The aim of this experiment is to study the displacement reaction between metals and their salts. THEORY Some metals are more reactive than others. In this experiment, a strip of metal is added to a solution of another. If the metal is more reactive than the metal in solution, this metal displaces (pushes out) the less reactive metal from the solution. PROCEDURE In this experiment, a strip of metal is added to a solution of another. Follow the step by step procedure as given below 1. Clean each of the metal strips with emery paper. 2. Using a teat pipette put some of the solution of a metal compound in four of the holes in the spotting tile. (Label this row with the name of the solution). 3. Do this for each solution of a metal compound. 4. Put a piece of each metal in each of the solutions. 5. Put a tick or a cross in your table to show if they have reacted. lassicxperiments 251 If the metal is more reactive than the metal in solution, this metal displaces (pushes out) the less reactive metal from the solution. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 61 IGCSE CHEMISTRY LABORATORY MANUAL OBSERVATION Record which metals react with the solutions. A table may be useful. Use a ✔to show reactivity and an✘to show no reaction. CONCLUSION The order of reactivity of these metals (from most to least reactive) is Most reactive ……………………… …………………….. …………………….. Least reactive GIS IGCSE CHEMISTRY (0620) …………………….. LAB MANUAL 62 IGCSE CHEMISTRY LABORATORY MANUAL EXPERIMENT 32– COMPARISON OF METALS REACTIVITY AIM OF THE EXPERIMENT: The aim of this experiment is to determine the activities of several metals based on observations of single replacement reactions. THEORY A single replacement reaction is one in which one element replaces another element in a compound. In the following reaction, zinc replaces the copper in copper(II) chloride: Zn(s) + CuCl2(aq) ZnCl2(aq) + Cu(s) If you were to observe this reaction, you would witness the decomposition of zinc metal and the simultaneous formation of copper metal. This reaction occurs because zinc is more active than copper, therefore zinc can replace copper in an aqueous compound. Very active metals can also replace hydrogen in water or acids, producing hydrogengas: Na(s) + H2O(l) NaOH(aq) + H2(g) In the above equation, sodium has replaced the hydrogen in water. However, the equation is not balanced. The balanced equation is: 2Na(s) + 2H2O(l) 2NaOH(aq) + H2(g) The products of single replacement reactions can be predicted by looking at the Activity Series of Metals. The Activity Series orders metals (and hydrogen) from most active to least active. Elements which are higher in the activity series can replace elements which are lower. In this experiment, you will be determining your own activity series based on your observations of single replacement reactions. You will check your results against the activity series given in your textbook. MATERIALS Copper metal Zinc metal Magnesium metal 8 test tubes Test tube rack Towel or sandpaper 0.1M-0.5M Solutions of: Copper(II) sulfate Zinc chloride Magnesium chloride Potassium chloride Lead(II) nitrate Silver nitrate PROCEDURE Obtain eight clean test tubes in a rack and label them A-H. In each of the test tubes, you are to combine 1-2 ml of a solution with a small piece of metal. This experiment is qualitative, so you do not need to measure the amounts of solution or metal that you use. However, it is helpful to polish the metals (to remove any corrosion) with a towel, sandpaper or steel wool before you begins. GIS IGCSE CHEMISTRY (0620) LAB MANUAL 63 IGCSE CHEMISTRY LABORATORY MANUAL TEST TUBE CONTENTS: CAUTION!! LEAD IS HARMFUL IF INGESTED!! WASH YOUR HANDS AFTER LAB!! A. Lead(II) nitrate solution + Copper metal B. Silver nitrate solution + Copper metal C. Copper(II) sulfate solution + Zinc metal D. Lead(II) nitrate solution + Zinc metal E. Magnesium chloride solution + Zinc metal F. Zinc chloride solution + Magnesium metal G. Sodium chloride solution + Magnesium metal H. Potassium chloride solution + Magnesium metal Record your observations for each test tube, particularly noting any color change on the surfaces of the metals. OBSERVATIONS Test tube A Observation B C D E F G H RESULT AND DISCUSSIONS The order of reactivity of these metals (from most to least reactive) is Most reactive ……………………… …………………….. Least reactive GIS IGCSE CHEMISTRY (0620) …………………….. LAB MANUAL 64 IGCSE CHEMISTRY LABORATORY MANUAL Write and balance the equations for the eight reactions that you observed,rememberingto indicate physical states. A. ………………………………………………………………………………………………… ………………………………………………………………………………………………… B. ………………………………………………………………………………………………… ………………………………………………………………………………………………… C. ………………………………………………………………………………………………… ………………………………………………………………………………………………… D. ………………………………………………………………………………………………… ………………………………………………………………………………………………… E. ………………………………………………………………………………………………… ………………………………………………………………………………………………… F. ………………………………………………………………………………………………… ………………………………………………………………………………………………… G. ………………………………………………………………………………………………… ………………………………………………………………………………………………… H.……………………………………………………………………………………………… ………………………………………………………………………………………………… GIS IGCSE CHEMISTRY (0620) LAB MANUAL 65