Titanium: Atomic Structure, Properties & Alloys
advertisement

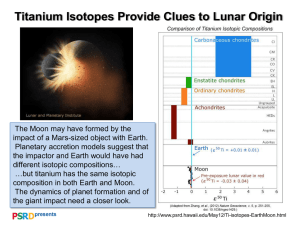
ATOMIC STRUCTURE OF TITANIUM Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, specific gravity of 4.54, with a valence of 2, 3, or 4. Pure titanium is a lustrous white metal with low density, high strength, and high corrosion resistance. It is resistant to dilute sulfuric and hydrochloric acids, moist chlorine gas, most organic acids, and chloride solutions. Titanium is only ductile when it is free of oxygen. Titanium burns in air and is the only element that burns in nitrogen. Titanium is dimorphic, with the hexagonal a form slowly changing to the cubic b form around 880°C. The metal combines with oxygen at red heat temperatures and with chlorine at 550°C. Titanium is as strong as steel, but it is 45% lighter. The metal is 60% heavier than aluminum, but it is twice as strong. Titanium metal is considered to be physiologically inert. Pure titanium dioxide is reasonably clear, with an extremely high index of refraction and an optical dispersion higher than that of a diamond. Natural titanium becomes highly radioactive upon bombardment with deuterons. TITANIUM ALLOY MADE OF Titanium is considered to be one of the strongest metals. Its strength, heat, water and salt resistance, and its light weight make it the ideal metal for a variety of applications. These applications range from jewelry and dental implants to airplanes and ships. Pure titanium is strong and corrosive resistant. Titanium alloys retain the same strength and corrosion resistance, but takes on the greater flexibility and malleability of the metal it is combined with. Titanium alloys, therefore, have more applications than pure titanium. There are six grades of pure titanium (grades 1,2,3,4,7 and 11) and 4 varieties of titanium alloys. Titanium alloys typically contain traces of aluminum, molybdenum, vanadium, niobium, tantalum, zirconium, manganese, iron, chromium, cobalt, nickel, and copper.