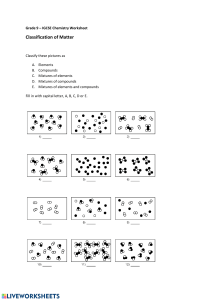
23.01.2025, 10:56 Elements, compounds, mixtures. | Blooket Blooket | Question Set Name Date Class Elements, compounds, mixtures. 1. Which of the following is an element? a) Water b) Carbon dioxide c) Iron d) Sugar 2. Which of the following statements is true for compounds? a) Compounds contain only one type of atom. Compounds always have a variable c) composition. b) Compounds can be separated by physical methods. Compounds are formed by the d) chemical combination of two or more elements. 3. Which of the following substances is a mixture? a) Table salt (NaCl) b) Water c) Air d) Methane (CH₄) 4. Which of the following is NOT a property of compounds? Compounds can be separated by physical methods. a) Compounds have a fixed composition. b) Compounds can be broken down into c) elements only by chemical methods. Compounds have new properties, d) different from the properties of the elements. 5. How are compounds formed? By a chemical reaction between two or more elements. a) By mixing elements. b) c) By cooling elements. d) By separating mixtures. 6. Which of the following is a compound? a) Sodium oxide b) Air c) Copper d) Iron filings 7. Which of the following substances is a mixture? a) Copper b) Water c) Steel alloy d) Aluminum dioxide https://dashboard.blooket.com/set/674f34ad36d2636d15146091/print?images=true&description=true 1/3 23.01.2025, 10:56 Elements, compounds, mixtures. | Blooket 8. Which characteristic distinguishes a mixture from a compound? a) A mixture always has a fixed composition. b) A mixture undergoes a chemical reaction. c) Components of a mixture can be separated by physical methods. d) A mixture has new properties different from its components. 9. Which of the following statements is true for elements? a) Elements can be broken down into simpler substances. b) Elements contain atoms of only one type. c) Elements can be separated by physical methods. d) Elements are formed during chemical reactions. 10. Which of the following is a property of compounds? a) Compounds can be separated by filtration. b) Compounds have a variable composition. c) Compounds are formed by chemical reactions. d) Compounds retain the same properties as their elements. 11. Which of the following is an element? a) Helium b) Salt c) Water d) Ammonia (NH₃) 12. Which of the following represents a chemical compound? a) Copper wire b) Foil c) Iron and sulfur mixed together d) Water 13. Which statement is true for mixtures? a) Substances in mixtures retain their properties. b) Substances in mixtures can be separated by physical methods. c) Substances in mixtures are chemically combined. d) Mixtures always have a fixed composition. 14. How do the properties of elements change when they form a compound? a) They remain the same as in the original elements. c) They disappear completely. b) They change and acquire new properties. d) They always become less stable. https://dashboard.blooket.com/set/674f34ad36d2636d15146091/print?images=true&description=true 2/3 23.01.2025, 10:56 Elements, compounds, mixtures. | Blooket 15. Which of the following is a characteristic property of a compound? Its components are physically combined. b) Its components can be separated by filtration. c) It always has a fixed composition. d) Its components can be seen with the naked eye. a) 16. Why is a mixture easier to separate than a compound? a) Mixtures involve chemical bonding. c) Components of mixtures are physically combined and retain their properties. b) Mixtures have a fixed composition. d) Compounds cannot be separated. 17. What is an element? a) A substance that consists of only one type of atom. b) A substance that contains two or more different atoms bonded together. c) A combination of substances that can be separated physically. d) A compound that contains a metal and a non-metal. b) A chemical combination of two or more elements. 18. What is a mixture? a) A substance that cannot be separated physically. c) A substance with a fixed composition. A physical combination of two or more d) substances that retain their individual properties. https://dashboard.blooket.com/set/674f34ad36d2636d15146091/print?images=true&description=true 3/3