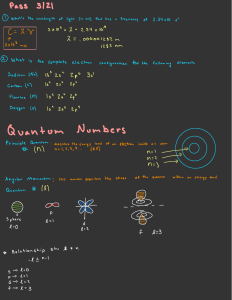
LESSON TOPIC: QUANTUM THEORY AND THE PHOTOELECTRIC EFFECT LEARNING GOALS We will learn what the difference is between particles and waves in terms of how they deliver energy and whether they exhibit an interference pattern in a double slit experiment. We will understand what all quantum objects display wave­ particle duality. We will learn about the photoelectric effect, and how that is evidence that light exhibits wave­particle duality. HW: Pg. 624 #1,2 Pg. 631 #1 ­ 3 LESSON TOPIC: QUANTUM THEORY AND THE PHOTOELECTRIC EFFECT In classical physics (the physics governing the everyday world), waves and particles are very distinct. Waves Particles ­ Deliver their energy continuously over time all over the screen (think of sound waves and water waves) ­ Deliver their energy in discrete amounts at specific locations on the screen ­ Exhibit an interference pattern when passed through a double slit ­ Do not exhibit an interference pattern when passed through a double slit 1 LESSON TOPIC: QUANTUM THEORY AND THE PHOTOELECTRIC EFFECT In the quantum world (including electrons, protons, atoms, and all electromagnetic radiation) the separation between particles and waves becomes blurred. All quantum objects exhibit both wave and particle properties. This phenomenon is classified as wave­particle duality. Electrons are passed through a double slit. Each one hits the screen in a discrete location (like a particle), and over time the buildup of electrons at different locations creates an interference pattern (like a wave). This is the trademark of all quantum objects: ­ They produce an interference pattern when passed through a double slit ­ The deliver energy in discrete amounts (called quanta) LESSON TOPIC: QUANTUM THEORY AND THE PHOTOELECTRIC EFFECT One experiment which puzzled physicists before the discovery of quantum theory was the photoelectric effect. When light is shone on a sample of metal, it is possible that an electron can be ejected with kinetic energy. In order to eject an electron, the work function must be overcome. The work function is the amount of energy that must be given to an electron for it to escape the metal. There are a list of different work functions on page 621 in eV 1 eV = 1.6 x 10­19 J There were two problems that could not be explained using classical physics. 1) No electrons were ejected if the light was below fo (called the threshold frequency). Increasing the intensity of the light (which would increase the energy of the wave in classical physics) did not have an effect on whether electrons were emitted. 2) The kinetic energy of the ejected electrons only depended on the frequency of the light. Again, increasing the intensity of the light had no impact on the energy of the ejected electrons. Albert Einstein contributed to solving the questions in the photoelectric effect (in fact, that's why he won his Nobel Prize in Physics) 2 LESSON TOPIC: QUANTUM THEORY AND THE PHOTOELECTRIC EFFECT Einstein proposed that light consisted of tiny particles called photons (packets of light energy with no mass) The energy of a photon can be calculated with the frequency of light used, and Planck's Constant E ­ Energy of the photon (J) h ­ Planck's Constant (6.63 x 10­34 J s) f ­ Frequency of light (Hz) This explained the photoelectric effect. Photons collide with electrons like a particle, delivering their energy to each electron. If the photon has an energy equal to the work function, electrons are ejected If the photon has a frequency higher than the threshold frequency, electrons escape and can have a maximum kinetic energy Increasing the intensity of the light increases the energy of the light, but it does so by increasing the total number of photons, not changing the energy of each photon. More electrons are emitted if the light is above the threshold frequency, but increasing intensity doesn't change the energy delivered to each electron. LESSON TOPIC: QUANTUM THEORY AND THE PHOTOELECTRIC EFFECT Because each metal has its own work function, it also has its own threshold frequency. The larger the work function, the higher frequency required (or lower wavelength.... due to the universal wave equation). Each line however has a slope of h and follows the linear equation Sample Problem: a) Calculate the energy of a photon and threshold frequency required to emit electrons from aluminum (W = 4.20 eV). b) Determine if blue light of wavelength 450 nm will emit electrons from aluminum. 3 LESSON TOPIC: COMPTON EFFECT, BLACKBODY RADIATION, MATTER WAVES LEARNING GOALS We will understand the different ways a photon interacts, including the Compton effect. We will know what blackbody radiation is, how it led to quantum theory, and how to use Wien's law to predict the maximum wavelength emitted. We will understand that all quantum objects like electrons have a wave nature, and how to determine the de Broglie wavelength for a particle. HW: Pg. 626 #1 ­ 3 Pg. 629 #1 ­ 3 Pg. 631 #4,6 Pg. 634 #1 ­ 4 LESSON TOPIC: COMPTON EFFECT, BLACKBODY RADIATION, MATTER WAVES Photons have energy (E = hf from last lesson), and they also possess momentum. There is evidence for this in the Compton effect ­ where a photon collides elastically with an electron and momentum is conserved. The photon gives some momentum and kinetic energy to the electron, which results in a decrease in the energy of the photon (its frequency decreases after the collision) How can an object with no mass (a photon) have momentum? OR OR 4 LESSON TOPIC: COMPTON EFFECT, BLACKBODY RADIATION, MATTER WAVES Sample Problem: Determine the momentum of a 1.0 GeV photon (energetic gamma ray) Overall, photons can interact with matter in 5 main ways: 1) A photon can reflect (when light hits a mirror, the photons collide elastically) 2) A photon may free an electron and be absorbed in the process (photoelectric effect) 3) A photon can lose energy and momentum in a collision with an electron. The photon still travels at the speed of light but loses frequency. (Compton effect) 4) A photon can be absorbed by an atom and elevate an electron to a higher energy level. When the electron returns to the lower energy state, it releases a photon equal to the difference in energy between the levels. 5) A photon can undergo pair creation ­ where a photon's energy is converted into two particles with mass. LESSON TOPIC: COMPTON EFFECT, BLACKBODY RADIATION, MATTER WAVES Blackbody radiation occurs when a black object emits EM radiation due to its temperature. Think about a stove element that changes colour as it gets hot. A blackbody is any object that absorbs all frequencies of light (and therefore can emit all frequencies of light). Classical physics predicted that the intensity of light emitted would increase towards infinity as the frequency increased. The prediction fell apart when compared to experimental results at the UV part of the spectrum ­ so it was called the ultraviolet catastrophe. Max Planck proposed a solution to the problem by assuming that energy was released in discrete amounts called quanta, rather than the continuous energy of a classical wave. This fit the data perfectly, but even Planck could not explain why it worked. The quanta of energy was proposed to be equal to hf (Einstein used this later) where h is Planck's constant. 5 LESSON TOPIC: COMPTON EFFECT, BLACKBODY RADIATION, MATTER WAVES As the temperature of the blackbody increases, the curve changes. The radiation intensity increases for all wavelengths (frequencies), and the peak of the curve shifts to lower wavelengths (higher frequencies). Note that all frequencies are emitted, the peak of the curve is what the object looks like in terms of colour. The λmax can be predicted mathematically using Wien's law. (This is also how ear thermometers determine your temperature. They detect the λmax your ear emits, and uses that to determine your body temperature) Sample Problem: Determine the colour that a 6500 K star would appear. LESSON TOPIC: COMPTON EFFECT, BLACKBODY RADIATION, MATTER WAVES Electrons, among other quantum objects, produce an interference pattern when they pass through a double slit. As a result, these objects have a wave nature, and in fact a wave length. This wavelength is called the de Broglie wavelength. Sample Problem: Determine the wavelength of an electron travelling at 1.0 x 106 m/s. 6 LESSON TOPIC: THE STANDARD MODEL LEARNING GOALS We will understand the problems with the planetary model of the atom, and the basis of the Bohr model. We will know what anti matter is, and similarities and differences it has to matter. We will understand what the standard model is, and how it describes what all matter is. HW: LESSON TOPIC: THE STANDARD MODEL The planetary model (Rutherford model) of the atom explains that electrons orbit the nucleus (much like planets orbit the sun) due to the electric force. Rutherford discovered that all of the positive charge in an atom was present in the centre of the atom (the nucleus) through the gold foil experiment. The problem with this model, is that an electron travelling in a circular path would be accelerating. As we learned in the last unit, this would cause it to emit electromagnetic radiation, which would decrease its energy. The electron's orbit would shrink until it collapsed into the nucleus, meaning the atom could not exist. 7 LESSON TOPIC: THE STANDARD MODEL The Bohr model of the atom uses a quantum mechanics approach. It looks at the wave nature of the electron, and says not all orbital radii are allowed ­ only those with a circumference equal to an integer number of standing wavelengths are allowed. 3 wavelengths fit into this orbit, so this radius of orbit is allowed. This would be the third energy level (n = 3 in chemistry). The number of wavelengths that fit the circumference correlate to the energy level of that shell. (i.e. 5 wavelengths means n = 5) In order for an electron to jump from a lower shell to a higher shell, the atom must absorb a photon with exactly the energy equal to the difference in the shells (corresponding to the wavelength of the light). When the electron returns to a lower state, it releases a photon of light with a wavelength that corresponds to the difference in energy between the shells. LESSON TOPIC: THE STANDARD MODEL Antimatter is a form of matter which has the same mass, but opposite charge as its matter counterpart. For example, the antimatter particle for an electron is the positron. For a proton, it's the antiproton, and for the neutron, the antineutron. Even through the neutron has no charge, the antimatter is still the 'opposite' charge when you see what the neutron is made up of. When matter collides with its antimatter counterpart, they annihilate, and turn their mass into energy (in the form of photons) in correspondence with E = mc2. 8 LESSON TOPIC: THE STANDARD MODEL The Standard Model is the most current model of the universe we have in terms of how matter and energy interact, and what everything is made up of. FERMIONS (Fundamental particles) Leptons Quarks (Electrons, muon, tauon, neutrinos) (Up, down, charm, strange, top, bottom) Combine to make 3 Quarks Baryons (Protons, Neutrons, etc) Hadrons 2 Quarks (1 quark, 1 antiquark) Mesons (pion, kaon, etc) LESSON TOPIC: THE STANDARD MODEL 9 LESSON TOPIC: THE STANDARD MODEL LESSON TOPIC: THE STANDARD MODEL The last types of particles included in the standard model are called bosons. Bosons act as the transmitter of force between two objects for 3 of the fundamental forces (electromagnetic, weak nuclear, and strong nuclear). Fermions are considered truly fundamental, and bosons are the way that the fundamental forces act between those fermions. Another boson called the Higgs boson is the last particle needed to make the standard model complete. It was discovered in the Large Hadron Collider in 2012. The Higgs boson is the particle which explains why fundamental particles have mass. 10 LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION LEARNING GOALS We will learn the basis behind Einstein's theory of Special Relativity, including its postulates and consequences. We will understand that relativistic effects are seen at high speeds, so most of them are undetectable in day to day life. We will know what happens to time for a clock that is moving at high speeds, compared to a stationary clock. HW: Pg. 585 #1 ­ 5 Pg. 587 #3,6 LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION Einstein's theory of special relativity was developed at a time that classical physics was exceptionally well understood (Newton's mechanics and Maxwell's electromagnetism). Unfortunately, there were a number of things not well explained (such as blackbody radiation, the photoelectric effect as we saw in quantum mechanics) and new theories in modern physics changed our understanding of the universe. Consider different frames of reference ­ inertial frame and non­inertial. In an inertial frame of reference all of the laws of physics you are used to apply (whether you are at rest or moving with constant velocity). In an accelerating frame of reference, you would need to invent forces to explain what you observe. If we have 2 inertial, yet different frames of reference, the laws of physics are equally valid in both, even if they see different observations. Observer 1 sees the ball travel only vertically whether he is at rest or moving at constant speed v. Observer 2, who sees observer 1's frame of reference moving at speed v relative to her, sees the ball travel in a parabolic path. They are both correct, as observer 2 will see the velocity that observer 1 sees for the ball, plus the velocity of the frame of reference. This is true for all objects such as baseballs. 11 LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION Electromagnetism did not seem to obey the fact that 2 observers in different inertial frames of reference should see the same laws of physics apply, and the movement of a frame of reference did not result in two observers measuring different speeds for light (like it would for a baseball). Einstein performed a thought experiment around a bar magnet and a coil of wire. When a bar magnet moves near a coil of wire, classical physics predicts a current due to an electric field near the magnet which pushes the charges through the wire. When the coil moves near the magnet, current still flows, but the reason from classical physics is that the moving charges feel a force due to the magnetic field. Einstein felt it illogical that changing which frame of reference was moving would change our understanding of what is happening. He proposed that electromagnetism, like Newtonian mechanics did not change when transformed between inertial reference frames. This lead him to two postulates (statements which are assumed to be true, leading to a theory). LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION Postulate 1: The Principle of Relativity The laws of physics are the same in all inertial frames of reference. No physics experiment can ever determine whether you are at rest or moving at a constant velocity. Postulate 2: The Speed of Light Principle There is at least one inertial frame of reference in which, for an observer at rest in this frame of reference, the speed of light, c, in a vacuum is independent of the motion of the source of the light. Separately these are not that extraordinary (or controversial). When considered together however, postulate 1 says that if postulate 2 is true for one inertial frame of reference, it must be true for all inertial frames of reference. In short, it means that an observer at rest OR at constant velocity will measure the speed of light to be c. (this is weird... it means if you drive past someone with your headlights on and are travelling at 0.5 c, you will see the light travelling at c, and so will an observer at rest on the side of the road.... weird...) This lead to the special theory of relativity: All physical laws are the same in all inertial frames of reference, and the speed of light is independent of the motion of the light source or observer in all inertial frames of reference. This also meant that things physicists considered to be the same for all observers (time, length, mass) were actually dependent on the observer measuring them, and in particular the relative speed between the two observers. 12 LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION The first affected quantity we will look at is time. The short story is that moving clocks move more slowly than clocks at rest. This effect increases with speed (and really only gets noticeable at high speeds). Look at a light clock, where a round trip for the light represents a 'tick' of the clock. Now if we consider two observers, one moving at a speed v along with the clock (so it would be stationary in their frame of reference) and a stationary observer watching the clock go past. Observer 1 is stationary relative to the clock, and so measures Δts for one 'tick'. Both observers see the light travel towards the top mirror, reflect, and travel back to the bottom mirror. Who measures a longer path for the light? What is the speed of light measured by each observer? Who measures a longer time for the 'tick' LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION To figure out the time that observer 2 measures (Δtm since the clock is moving relative to her with speed v) we need the distance the light travels and its speed. The light takes the red path in the picture to the left. Δtm ­ the time measured for an event that is moving relative to the observer Δts ­ the time measured for an event that is stationary relative to the observer (also called proper time) v ­ the relative speed between the two frames of reference 13 LESSON TOPIC: SPECIAL RELATIVITY AND TIME DILATION At low speeds, relativistic effects on time are minimal. At higher speeds, a moving clock will measure less time than a stationary clock (more time passes on Earth for example than does on a space ship passing by the earth at high speed) Sample Problem: On Earth, an astronaut has a pulse of 75.0 beats per minute. He travels into space in a spacecraft capable of reaching very high speeds. Determine the astronauts pulse as measured by observers on Earth when: a) The spacecraft travels at 0.10c b) The spacecraft travels at 0.90c LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM LEARNING GOALS We will learn the effects of high speed on the measurement of length between 2 points. We will understand how time dilation and length contraction work together to clarify apparent paradoxes in relativity. We will learn what happens to momentum (and mass) as objects travel at high speed. HW: Pg. 591 #1 ­ 3 Pg. 596 #1 ­ 4 14 LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM Just like time is not an absolute quantity measured the same by all observers, length also shows relativistic effects due to the fact that all observers in inertial frames of reference measure the speed of light to be c. Consider how the 2 observers would measure the length between points A and B on the ground in the picture to the left. The length is at rest relative to observer 2, so she would measure Ls (the proper length), and Δtm for the time that observer 1 travels from A to B. Observer 1 would read his clock as he passed point A, and then read it again when he passes point B. The time between the two measurements would be Δts as the clock is at rest relative to him. He would also measure Lm for the length as it is moving relative to him. Multiply both sides by v, substitute the expressions for length, and isolate for Lm Lm ­ the length of something moving relative to an observer Ls ­ the length of something at rest relative to an observer (proper length) LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM What this means, is if you travel at high speed, space contracts in the distance that you are moving. If you are travelling to the moon at high speed, the distance that you have to travel will decrease due to length contraction. Also, any objects you see on your way will contract in the direction that you travel. The faster you go, the more the length contracts. If we switch our observers and have observer 1 at rest, and observer 2 riding a metre stick at high speed towards observer 1, the picture is like this: Now the metre stick is at rest relative to observer 2, so she will measure it to be 1 metre long. The metre stick is moving relative to observer 1, so its length will contract and be shorter than a metre. (Also, observer 2 will contract, and will appear thinner since she contracts in that direction, but the same height as she does not contract in that direction) 15 LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM Sample Problem: An observer on Earth measures the length of a spaceship travelling at 0.700c to be 78.0 m long. Determine the proper length of the spacecraft. Muons are a sample of relativistic effects. They have a lifetime of 2.2 μs at rest, and travel at 0.99c. They are created in the upper atmosphere (4500 m above the Earth). The should decay well before they reach the ground, but are detected at the surface. How is that possible? LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM The twin paradox is an example of how relativity should be applied: Length contractions and time dilations should be considered together rather than separately. One twin is placed on a spacecraft, the other stays on Earth. The first twin travels to Sirius (8.6 ly away) at 0.90c and returns. The Earth twin would see the astronaut twin moving at high speeds and would conclude that he ages 8.3 years while she ages 19 years. The astronaut twin would say that the Earth twin is moving away at 0.90c and therefore she would age 8.3 years while he ages 19 years. Who is right? (This is the apparent paradox...) If you just consider time dilation without the context of length contraction, it is a paradox. However, if you consider length contraction as well, the astronaut would see the distance to Sirius contracted (as it's moving relative to him) and would only travel 3.7 ly. Essentially, the astronaut would experience the shorter time (8.3 years) due to the fact that he also experiences the shorter length. The Earth twin would see the ship travelling all 8.6 ly, and therefore would measure the longer time. The Earth twin would be correct. One observer measures a dilated time, the other observer measures a contracted length. 16 LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM High speeds also impact the momentum of an object. The momentum of an object can be shown to be Much like the equation for time dilation, the momentum increases significantly at high speeds. As objects approach a speed of c, the momentum increases to infinity. The other consequence of this effect, is the effect on mass. The rest mass is m, and the relativistic mass is larger at higher speeds (this is why momentum increases). The result is that as objects approach the speed of light, their mass (and therefore inertia) approaches infinity ­ which is why no object with mass will ever be able to get accelerated to the speed of light. LESSON TOPIC: LENGTH CONTRACTION, RELATIVISTIC MOMENTUM Sample Problem: In experiments to study the properties of subatomic particles, physicists routinely accelerate electrons to speeds close to the speed of light. Determine the following momentums for an electron travelling at 0.99c: a) Classical momentum b) Relativistic momentum 17 LESSON TOPIC: RELATIVISTIC KINETIC ENERGY, MASS ENERGY EQUIVALENCE LEARNING GOALS We will learn about mass­energy equivalence as predicted by Einstein's equation E=mc2 We will understand what the rest mass and rest energy of an object mean, and how to calculate them. We will know how to determine the total energy, rest energy, relativistic kinetic energy. HW: Pg. 591 #1 ­ 3 Pg. 596 #1 ­ 4 LESSON TOPIC: RELATIVISTIC KINETIC ENERGY, MASS ENERGY EQUIVALENCE Classically, doing work on an object can increase its kinetic energy, and therefore its speed. As we learned last lesson, no object with mass can reach the speed of light (due to its increasing mass, and therefore inertia). As a force continues to do work on an object, where does the energy go, if it isn't increasing the speed of the object? The answer is that it is increasing the mass of the object. This may sound weird at first, but it is true according to mass­energy equivalence (E = mc2). Energy and mass are the same thing, and you can convert one form into the other, where the speed of light squared is the conversion factor. It is this mass energy equivalence that allows the sun to produce energy (fusing hydrogen into helium results in less mass, therefore producing energy), nuclear power plants to function (having uranium undergo fission), and unfortunately nuclear weapons as well. Sample: Determine the amount of energy available if you could convert 1 kg entirely into energy. The daily energy consumption for all of Canada is about 2.4 x 1016 J. How long could this 1 kg power Canada? 18 LESSON TOPIC: RELATIVISTIC KINETIC ENERGY, MASS ENERGY EQUIVALENCE This equation came from our last lesson on relativistic momentum, where m is the rest mass of the object. The total energy of an object can be found using the relativistic mass, i.e. When the object is at rest, the denominator becomes 1, and therefore the total energy of the object is due to the rest mass of the object. The rest energy of the object is a property solely of its rest mass, and does not change with the motion of the object. At high speeds however, we know that it will have a higher total energy, so this 'extra' energy possessed by the object is its relativistic kinetic energy. Rearranging for Ek and substituting the equations for the total energy and rest energy we get the equation for the relativistic kinetic energy. Like all variables we look at, kinetic energy is similar to classical kinetic energy at low speeds, then becomes very large (and eventually infinity as v approaches c) LESSON TOPIC: RELATIVISTIC KINETIC ENERGY, MASS ENERGY EQUIVALENCE Sample Problem: An electron has a rest mass of 9.11 x 10­31 kg. Determine the following quantities relative to a laboratory frame of reference if the electron moves at 0.900c relative to the laboratory (in MeV): a) Its rest energy b) Its total energy c) Its kinetic energy 19