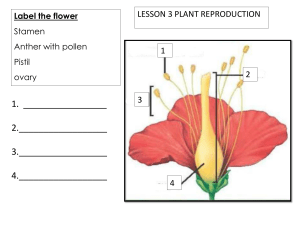
E BOOK Date Palm Biotechnology Protocols Volume II Germplasm Conservation and Molecular Breeding (Methods in Molecular Biology, 1638) 1st ed. 2017 Edition by Jameel M. Al-Khayri , S.Mohan Jain , D
advertisement

Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Contents PART I GERMPLASM IN VITRO CONSERVATION 1 Storage and Viability Assessment of Date Palm Pollen . . . . . . . . . . . . . . . . . . . . . . . 3 2 In Vitro Conservation of Date Palm Tissue Cultures . . . . . . . . . . . . . . . . . . . . . . . . 15 3 Cryopreservation of Date Palm Pro-Embryonic Masses Using the D Cryo-plate Technique. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25 4 In Vitro Cryopreservation of Date Palm Caulogenic Meristems. . . . . . . . . . . . . . . 39 5 In Vitro Conservation of Date Palm Shoot-Tip Explants and Callus Cultures Under Minimal Growth Conditions. . . . . . . . . . . . . . . . . . . . . 49 6 In Vitro Conservation of Date Palm Somatic Embryos Using Growth-Retardant Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61 7 Encapsulation of Date Palm Somatic Embryos: Synthetic Seeds. . . . . . . . . . . . . . . 71 PART II MOLECULAR ANALYSIS OF IN VITRO CULTURES 8 Evaluation of Clonal Fidelity of Micropropagated Date Palm by Random Amplified Polymorphic DNA (RAPD). . . . . . . . . . . . . . . . . . . . . . . . . . 81 9 Molecular Identification of Fungal Contamination in Date Palm Tissue Cultures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91 PART III GENETIC DIVERSITY AND CULTIVAR IDENTITY 10 Genetic Diversity Analysis of Date Palm Using Random Amplified Polymorphic DNA (RAPD) and Inter-Simple Sequence Repeat (ISSR) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105 vii Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com viii Contents 11 Date Palm Genetic Diversity Analysis Using Microsatellite Polymorphism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113 12 Assessing Date Palm Genetic Diversity Using Different Molecular Markers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 125 13 Molecular Analysis of Date Palm Genetic Diversity Using Random Amplified Polymorphic DNA (RAPD) and Inter-Simple Sequence Repeats (ISSRs) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 143 14 Determining Phylogenetic Relationships Among Date Palm Cultivars Using Random Amplified Polymorphic DNA (RAPD) and Inter-Simple Sequence Repeat (ISSR) Markers . . . . . . . . . . . . . . . . . 153 15 Genotyping and Molecular Identification of Date Palm Cultivars Using Inter-Simple Sequence Repeat (ISSR) Markers . . . . . . . . . . . . . . . 173 16 Molecular Identification of Date Palm Cultivars Using Random Amplified Polymorphic DNA (RAPD) Markers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 185 PART IV GENDER IDENTIFICATION 17 Early Sex Identification in Date Palm by Male-Specific Sequence-Characterized Amplified Region (SCAR) Markers . . . . . . . . . . . . . . . . . 199 18 Gender Identification in Date Palm Using Molecular Markers . . . . . . . . . . . . . . . . 209 19 Development of Sex-Specific PCR-Based Markers in Date Palm . . . . . . . . . . . . . . 227 20 Date Palm Sex Differentiation Based on Fluorescence In Situ Hybridization (FISH) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 245 PART V GENOMICS 21 Characterization and Amplification of Gene-Based Simple Sequence Repeat (SSR) Markers in Date Palm . . . . . . . . . . . . . . . . . . . . . . . 259 22 Mitochondrial Molecular Markers for Resistance to Bayoud Disease in Date Palm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 273 Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Contents ix 23 Analysis of Expressed Sequence Tags (EST) in Date Palm. . . . . . . . . . . . . . . . . . . . 283 24 Development of Genomic Simple Sequence Repeats (SSR) by Enrichment Libraries in Date Palm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 315 25 MicroRNA Expression in Multistage Date Fruit Development . . . . . . . . . . . . . . . 339 PART VI PROTEOMICS 26 Proteome of Abiotic Stress Tolerance in Date Palm . . . . . . . . . . . . . . . . . . . . . . . . . 355 27 Electrophoresis-Based Proteomics to Study Development and Germination of Date Palm Zygotic Embryos . . . . . . . . . . . . . . . . . . . . . . . . . . . 365 28 Date Fruit Proteomics During Development and Ripening Stages . . . . . . . . . . . . 381 Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 399 Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Chapter 1 Storage and Viability Assessment of Date Palm Pollen Abstract Pollen storage and viability are very important for pollination, breeding, biodiversity, biotechnology, conservation, and other biological and non-biological studies of the date palm. Optimizing procedures and duration of storage are important for effective and long-term date palm pollen storage and viability. Here we describe pollen storage methods, such as room temperature (25–30 C), refrigeration (4 C), storage at 4 C in desiccators, deep freezer (20 C), and cryopreservation (196 C). Based on pollen viability by staining and in vitro germination methods, cryopreservation is the best method for long-term storage without any significant effect on pollen viability (75–84%); however, the percentage of pollen viability depends on the storage period. Key words Acetocarmine, In vitro germination, Pollen storage, Pollen viability 1 Introduction Pollen viability affects pollen germination and delivers the nuclei to the embryo sac for compatible fertilization [1]. Date palm is a dioecious plant, and cross-pollination is the general rule. Hence, date palm pollen storage is an expedient practice; however, storage methods and pollen viability are subjects worthy of research. Pollen can retain its viability much longer under dry conditions. Pollen is collected at anthesis and stored in stoppered bottles [2], refrigerated or at room temperature for 4 weeks without losing viability and performance [3]. Various pollen storage methods indicate that short-term storage (230 days) is the best in desiccators, whereas freeze-drying is effective for long-term storage [4]. Previously, viability of pollen grains stored at various conditions has also been examined at room temperature (25–30 C), in a refrigerator (3–4 C) [5], at 4 C in desiccators (anhydrous calcium chloride) [4], deep freezer (20 C) [6], and cryopreservation at 196 C [7]. Furthermore, pollen viability can be determined with Alexander’s stain, fluorescein diacetate (FDA), acetocarmine or in vitro Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 4 Maryam et al. pollen germination [8]. Acetocarmine is widely used to test the germination rate of stored pollen grains on germination [9]; however, viability of fresh and stored pollens (1–12 months) can also be evaluated by applying different storage methods and germination tests, as different storage conditions had no apparent effect on pollen viability as tested with acetocarmine. Different media are used to compare stored and fresh pollen germination rates and tube elongation indicating significant decrease in pollen tube length in stored pollen grains [5]. The pollen germination rate was poor (8.5%) when stored at 0 C for 6 weeks; however, the best temperature for pollen tube elongation was 30 C [10]. Phoenix pollen has been stored cryogenically by immersing pollen, wrapped in aluminum foil, in liquid nitrogen (196 C) for long-term storage [7, 11]. Pollen viability gradually decreases although its shelf life is enhanced through low-temperature storage. Germination testing is highly reliable to determine pollen viability [12, 13]. On the other hand, pollen capacity to fertilize the ovule and set fruit is considered a measure of date fruit production [14]. In this chapter, we describe pollen storage and viability testing procedures, which are important for date palm pollination and production, due to asynchronized inflorescence emergence and anthesis between male and female date palms. 2 Materials 2.1 Plant Material 2.2 Reagents and Supplies Pollen grains collected from mature spathes of date palm Halawy cv. (see Note 1). 1. Pollen waxing: 200 g paraffin wax, paraffin film (see Note 2). 2. Pollen germination medium: 0.42 g/L Ca(NO3)2.2H2O (see Note 3), 0.2 g/L H3BO3, 0.1 g/L KNO3, 0.22 g/L MgSO4.7H2O, 200 g/L sucrose, and 10 g/L agar in distilled water. 3. Acetocarmine staining solution (1%): 100 mL of 45% glacial acetic acid, 1 g carmine powder. 4. Cryopreservation: Liquid nitrogen (196 C). 2.3 Equipment 1. Instruments: Hot air oven, magnetic stirrer, weighing balance, pH meter, autoclave, refrigerator, deep freezer, microscope, desiccator, and thermometer. 2. Glassware: Cryotubes (1 mL), glass test tubes (10 mL), beakers (50, 500 and 1000 mL), Petri plates, and measuring cylinders (50, 100, 500 and 1000 mL). 3. Supplies: Parafilm, spatula, pharmaceutical capsules (1 mL), and wax. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Storage and Viability of Pollen 3 Methods 3.1 Pollen Collection 5 1. Select mature spathes of male date palm, which are either ready to open or already opened, and cut at the base (see Note 4). 2. Place cut spathes for 2–3 days in the dark at 25–30 C and 30–40% relative humidity for anther dehiscence. 3. Separate pollens by shaking the inflorescence strands on a paper sheet (Fig. 1). 4. Dry pollen at room temperature and sieve (40 mesh ¼0.42 mm pore size) to separate petals and other inert material. 3.2 Pollen Storage 1. Encapsulate pollen for long-term storage in pharmaceutical capsules as follows: (a) Take 200 g paraffin wax in 250-mL beaker and heat in oven at 45 C for 2 min. (b) Dip pollen-filled capsules in melted wax (35–40 C). (c) After 8–10 min, transfer each waxed capsule in a Petri plate, and wrap in paraffin film. Fig. 1 Collecting (a) and drying (b) of date palm pollen grains after dehiscence Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 6 Maryam et al. Fig. 2 Storage of pollen grains at different storage temperature after waxing 2. Place capsules in labeled zipped polythene bags. Store bags under following storage conditions for up to 12 months (Fig. 2): (a) Room temperature (25–30 C). (b) Refrigerator (4 C). (c) Storage at 4 C in the desiccators (see Notes 5 and 6). (d) Deep freezer (20 C). (e) Cryopreservation (196 C) (see Note 7). 3. For cryopreservation, transfer the dehydrated pollen into cryotubes (0.56 g/mL), close the lid, and immerse in liquid nitrogen for 15 min. 4. Store the cryotubes in a freezer at 196 C. 3.3 Pollen Viability Tests 3.3.1 Pollen Germination Test 1. Soak glassware in liquid detergent for 1 h and thoroughly wash with hot water. Rinse the glassware with double-distilled water, and air-dry before use (see Note 8). 2. Prepare the germination medium as follows: (a) Weigh the chemicals for germination medium individually (for 1 L medium), and put in a 500-mL glass beaker. Add 300 mL distilled water to the beaker, and place on magnetic stirrer for dissolution of chemicals (see Note 9). (b) Adjust the pH of medium to 5.7 prior to the addition of agar. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Storage and Viability of Pollen 7 Fig. 3 Thawing of pollen grains after storage at 20 C (c) Weigh 10 g agar, put it in 100 mL glass beaker, add 100 mL distilled water, and boil. (d) Pour 100 mL boiled agar and 300 mL solution of salts and sucrose into a 1000 mL conical flask. Mix it vigorously; close the flask with aluminum foil, and autoclave at 121 C for 20 min. (e) In a laminar hood cabinet, pour the medium in sterile 50 mm diameter Petri plates and allow to solidify. 3. Sterilize camel hair brush for spreading pollen on the germination medium. 4. Thaw the frozen and cryopreserved stored pollen grains by swirling tubes in water bath, at 45 C, until the ice melts (Fig. 3, see Note 10). 5. After thawing, dust the stored pollens with a camel hair brush in Petri plates containing pollen germination medium under a laminar airflow cabinet to minimize the risk of contamination (Fig. 4). 6. Seal the plates with paraffin film, and incubate in a growth room at 25 2 C and 16-h photoperiod of 100 μmol/m2/s. 7. Count the number of germinated and total number of pollen grains (see Note 11) in each visible field of the microscope using 200 magnification within 1–24 h (Fig. 5, see Notes 12–14). 8. Calculate pollen germination percentage by using the following formula (see Note 15). Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 8 Maryam et al. Fig. 4 Culture of pollen grains on the germination medium Fig. 5 Viability of 12-month stored pollen grains tested by acetocarmine staining (magnification 200) [NV Non-viable, V Viable] Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Storage and Viability of Pollen Germination ð%Þ ¼ 9 Number of germinated pollen grains 100 Total number of pollen grains 9. Measure pollen tube length (see Note 16) at successive intervals under a microscope equipped with an eyepiece and stage micrometer using 200 magnification (see Note 17). 3.3.2 Pollen Staining Test 1. Prepare the acetocarmine solution for pollen staining as follows: (a) Boil 100 mL 45% glacial acetic acid in a glass beaker. (b) Weigh 1 g carmine powder, transfer to boiling glacial acetic acid, and boil gently for 5 min in a reflux condenser. Shake well and filter when cool. (c) Store the solution in a tightly closed screw-capped dark glass bottle under cool (4 C) and dry conditions (see Note 18). 2. Place one drop of 1% acetocarmine solution on a glass slide. 3. Place a drop of pollen suspension on glass slide, put a cover slip on top of it, and gently press the cover slip to remove extra stain with tissue paper/filter paper (see Note 19). 4. Examine stained and unstained pollens under the microscope at 200 magnification (Fig. 6, see Note 20). Fig. 6 In vitro pollen germination after 12-month storage (20 C) incubated at 28 C (magnification 200) Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 10 Maryam et al. 5. Calculate stained pollen percentage by using the following formula. Staining ð%Þ ¼ 4 Number of stained pollen grains 100 Total number of pollen grains Notes 1. The age of the date palms in this study was about 30 years. 2. Wax the capsules, and wrap with paraffin film as a precaution to avoid possible capsule cracking that may occur due to dryness. 3. Calcium nitrate is also important for date palm pollen in vitro germination. Application of certain concentrations of calcium nitrate in culture media increased the pollen germination rates, but its higher concentration could decrease it. Pollen germination and pollen tube growth are regulated by transferring inorganic Ca2+ and K+ ions across the plasma membrane. Calcium also plays a role in determining the direction of pollen tube growth. For in vitro pollen germination and pollen tube growth, boron ion (B+) is one of the essential factors. 4. Select and cut a male spathe, either already split open or about to split open, and has brown color and soft texture. To prevent wind or bees from causing loss of pollen, it is recommended that the freshly opened spathe be cut early in the morning. 5. Before placing pollen in desiccators, fill it with new silica gel for effective moisture control. 6. Desiccators are used to store dried samples in dry atmosphere by using desiccants like silica gel, whereas pollen storage in a refrigerator at low temperature slows down metabolic processes and does not lead to pollen drying. 7. Ultra-low temperature (196 C) is the best for long-term storage of date pollen without any deteriorating effect on viability. 8. Prepare all solutions using distilled water. Chemicals and reagents should be of analytical grade. 9. Gradually increase the speed of stirrer for uniform movement of magnet, otherwise it will move abruptly and the beaker may break. If chemicals do not dissolve, increase the temperature up to 40 C. 10. Do not expose the frozen pollen capsules directly to hot water, but thaw them using glass test tubes in a beaker filled with hot water. Swirl these tubes in hot water at 65 C for 10 min. Then place the capsules on Petri plates, remove the paraffin and Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Storage and Viability of Pollen 11 wax using tissue paper, and collect the pollen on labeled Petri plates. 11. Pollen germination percentage during incubation period at 3 and 24 h ranges from 15 to 85%, respectively. 12. Pollen incubation on culture media up to 24 h ensures maximum germination. 13. A pollen grain is considered germinated when the pollen tube length is equal to or greater than the diameter of the grain. 14. Pollen culture medium can become contaminated if incubated for duration longer than 24 h. Although sterilized culture media and sterile Petri plates are used, the culture media can become contaminated when Petri plates are opened during microscopic studies. 15. Count germinated and total pollen in at least five microscopic fields, add accordingly, and then calculate the pollen germination percentage according to the given formula. For example, in the visible field of microscope, the total number of pollen are 90, out of which 75 pollen germinated, then the percent pollen germination is 83%. 16. Pollen tube length varies from 44 to 196 μm at 3 and 24 h, respectively. 17. Place the ocular micrometer inside the body tube of the microscope by unscrewing the eyepiece of the microscope. Then place the stage micrometer on the stage of the microscope, and focus the scale for calibration. The stage and ocular micrometer scales must be in the same direction. Compare and count the number of divisions of the point where both scales coincide with each other under the microscope with 40 object and 10 eyepiece magnification. Each of the 100 parts of the stage micrometer scale represents 0.01 mm, i.e., 10 μm (Fig. 7). For example, if 50 ocular micrometer scales are equal to 65 scales of stage micrometer, then 50 scales of ocular micrometer ¼ 0.65 mm 1 scale of ocular micrometer ¼ 0.65/50 ¼ 0.013 mm ¼ 13 μm After calibration, place the sample slide on the stage under the microscopic object for pollen tube length measurement. It is already determined that each scale of ocular micrometer is equal to 13 μm at 40 object and 10 eyepiece magnification of the microscope. The length of the pollen tube will be the number of scales multiplied by 13 μm. 18. Since the solution contains 45% glacial acetic acid, it is recommended to store the solution in a tightly closed dark screw cap glass bottle, in cool, dry, well-ventilated place away from inflammable material. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 12 Maryam et al. Fig. 7 Illustration of ocular and stage micrometer used for pollen tube measurement 19. Place cover slip gently and press it slightly with care to avoid air bubbles. 20. The pollen grains appearing normal and stained red are considered viable, whereas weakly stained or colorless are recorded as non-viable. The viability of stained pollen varies from 65 to 87% at storage periods of 1 and 12 months, respectively. Acknowledgment This work was supported by the Higher Education Commission, Pakistan and Plant Tissue Culture Cell, University of Agriculture, Faisalabad 38040, Pakistan. References 1. Shivanna KR, Linkens HF, Cresti M (1991) Pollen viability and pollen vigor. Theor Appl Genet 81:38–42 2. Ibrahim AMF, Kobbia EL (1998) Influence of the storage periods on pollen viability of four date male types. Alex J Agric Res 43:247–257 3. Gupta MR, Thatai SK (1980) Storage of date pollen. Punjab Hort J 20:211–214 4. Boughdeiri L, Boungaga N (1991) Storage of date palm pollen (Phoenix dactylifera L.). 1. preliminary results. Ann Sci Nat 11:119–124 5. Maryam JMJ, Fatima B, Haider MS, Naqvi SA, Nafees M et al (2015) Evaluation of pollen viability in date palm cultivars under different storage temperatures. Pak J Bot 47:377–381 6. Shaheen MA (1983) Identification of some seedling male date palms by pollen ultrastructure. J Coll Agric King Saud Univ 5:137–142 7. Ateyyeh AF (2012) Effect of storage method on date palm and pistachio pollen viability. Jordan J Agric Sci 8:573–582 8. Silva ED, Rodrigues LR, Mariath JEA (2011) Contradictory results in pollen viability determination of Valeriana scandens L. Geneconserve 40:234–242 9. Shaheen MA (2004) Evaluation of date palm males using pollen viability and ultrastructure. Acta Hortic 632:37–43 10. Al-Helal AA, Basalah MO, Muhammad S (1988) Effect of storage and temperature on pollen germination and range of pollen tube elongation of date palm (Phoenix dactylifera L.) Phyton Buenos Aires 48:119–122 Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Storage and Viability of Pollen 11. Tisserat B, Ulrich JM, Finkle BJ (1981) Survival of Phoenix pollen grains under cryogenic conditions. J Crop Sci 23:461–470 12. Bolat J, Pirlak L (1999) An investigation on pollen viability, germination and tube growth in some stone fruits. J Agric Forest 23:383–389 13 13. Rodriguez-Riano T, Dafni A (2000) A new procedure to assess pollen viability. Sex Plant Reprod 12:241–244 14. Boughediri L, Bounaga N (1987) In vitro germination of date pollen and its relation to fruit set. Date Palm J 5:120–127 Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name.