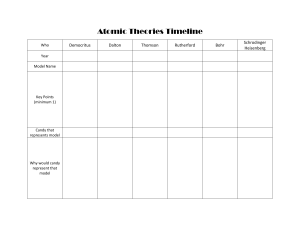
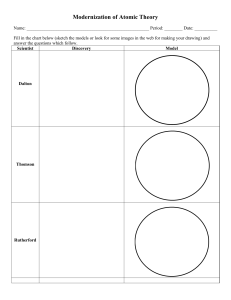
Name: Surname: History of Atomic Theory (Worksheet) Q.1. Fill in the blanks with suitable words. a) The word atom comes from a Greek word that means _______________. b) Dalton deduced that all elements are composed of _____________. c) ______________ discovered that there were small particles inside the atom. d) According to the Bohr Model, electrons travel around the nucleus in well-defined paths called _______________. e) Erwin Schrodinger describes the _________________ in 1926. Q.2. Write T or True if the statement is true; write F or False if the statement is false. _________ 1. Neutrons orbit the nucleus. _________ 2. The atomic theory has changed over time. _________ 3. Protons are positive, electrons are negative, neutrons have no charge. Q.3. Label the major ideas for atomic models. Q.4. Who was the first scientist to discover the existence of an atom? a) Democritus b) J.J. Thomson c) Dalton d) Niels Bohr Q.5. Who first proposed an atomic theory based on scientific knowledge? a) Ernest Rutherford b) J.J. Thomson c) Dalton d) Niels Bohr Q.6. Who first discovered electrons? a) Ernest Rutherford c) Dalton b) J.J. Thomson d) Niels Bohr Q.7. Who first discovered neutrons? a) Erwin Schrodinger c) Niels Bohr b) J.J. Thomson d) James Chadwick Q.8. Who first discovered protons? a) Ernest Rutherford c) Dalton b) J.J. Thomson d) Niels Bohr Name: Surname: Atomic Structure Counting Subatomic Particles Name__________________________ Date_____________ Name of the Element 23 Silicion 24 25 26 Sodium 27 28 Selenium 29 30 31 Silver 32 33 Tellurium 34 35 Antimony 36 37 Bismuth 38 39 Bromine 40 41 Tungsten 42 43 Manganese 44 Symbol Atomic Mass Number Number Z A 14 82 Protons Neutrons A-Z Electrons 73 108 12 45 45 71 28 207 11 35 34 Ti 48 47 56 52 108 137 128 V 51 Cs w Cr 35 48 74 24 81 23 28 23 18 22 18 209 133 80 112 55 110 52 25 Mo 42 77 122 Ar 83 22 54 36 96 30 25 Name: Surname: Periodic Table Scavenger Hunt Using a copy of the periodic table of elements, find the atomic symbol for the following elements 1. Barium 4. Gold __________ __________ 7. Potassium 10. Radon __________ __________ 13. Lanthanum __________ 2. Lead __________ 3. Nitrogen __________ 5. Strontium __________ 6. Lithium __________ 8. Chlorine __________ 9. Cesium __________ 11. Helium __________ 12. Berkelium 14. Bismuth __________ 15. Krypton __________ __________ Write the name of the element represented by the following atomic symbols 34. Sb _____________________________ 35. C _____________________________ 36. Mn _____________________________ 37. Al _____________________________ 38. Ge _____________________________ 39. Mt _____________________________ 40. Ag _____________________________ 41. Ne _____________________________ 42. P _____________________________ 43. Hg _____________________________ You will need a periodic table of elements to complete this activity 1. Of the first 103 elements, how many have just one letter in their symbols? _______________ 2. How many have two letters in their symbols? _____________ Name: Surname: 3. Name some elements that have a one-letter symbol that is also the first letter of the word. _______________________ _______________________ _______________________ _______________________ _______________________ _______________________ 4. Name some elements that have two-letter symbols that are the first letter of the word. _______________________ _______________________ _______________________ _______________________ _______________________ _______________________ 6. Why are their symbols different from their names? ____________________________________________________________________________ 7. Name some elements that are named after planets _______________________ _______________________ _______________________ 8. Name some elements after scientists _______________________ _______________________ _______________________ 9. Name some elements named after a city, a state, a country, or a university _______________________ _______________________ _______________________ Name: Surname: