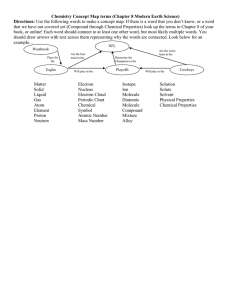
Structure of Water • H2O molecule has 8 electrons ( 6 from O2 & 1 from each H) • Two covalent bonds (one electron from O2 and one from H) • H-O-H angle is 105°, tetrahedron shape due to the greater repulsion between the non-bonding electron pairs on the O2 atom which pushes the H atoms closer together. • O2 is more electronegative than H. So H atoms has regions of +ve charge & O2 has regions of –ve charge. • Polar molecule