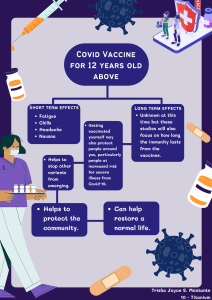
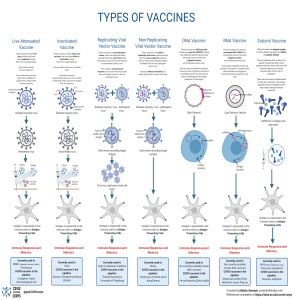
INTRODUCTION TO MODERN BIOLOGY Biology • • • “Bios” – life “Logos” – study the science of life and living organism Organisms • a living en ty consis ng of one cell e.g. bacteria, or several cells e.g. animals, plants and fungi. Main Concepts • • the cell is the basic unit of life. genes (consisting of DNA or RNA) are the basic unit of heredity. • evolution on accounts for the unity and diversity seen among living organisms. • all organisms survive by consuming and transforming energy. Three Main Divisions • • • Botany o Study of the plants. Zoology o Study of the animals. Microbiology o Study of the microorganisms. Different Branches • • • • • • • • • Morphology o deals with the study of form and structures of living organisms like shape, color, etc. Anatomy o the study of internal structures, like bones and organs. Histology o study of tissues with the help of microscope. Cell Biology o the study of the structures and functions of cells o also deals with the study of cell division Physiology o Deals with the study of the functions of with the study of the functions of different parts of living organisms. Molecular Biology o Deals with a study of the molecules of me for example water proteins lipids and nucleic acids. Genetics o the study of genes and their roles in inheritance o Inheritance – the transmission of characters from one generation to the other. o Gene structure and function, variation, and distribution are studied within the context of the cell, the organism (e.g. dominance), and within the context of a popula on. Embryology o the study of the development of an embryo to new individual. Taxonomy o the study of the naming and classification of organisms into groups and subgroups. • Paleontology o Study of fossils which are the remains of organisms. • Environmental Biology o the study of the interactions between organisms and their environment. • Sociobiology o this branch deals with the study of social behavior of the animals to make society. • Parasitology o Deals with the study of parasites. o Parasites – the organisms that take food and shelter from living hosts and in return harm them. • Biotechnology o deals with the practical application of living organisms to make substances for the welfare of mankind. o utilizes biological systems, living organisms or parts of this to develop or create different products. • Immunology o study of the immune system of animals which defends the against invading microbes. • Entomology o study of insects. • Pharmacology o study of drugs and their effects on human body. Microbiology • • the study of all living organisms that are too small to be visible with the naked eye. includes bacteria, archaea, viruses, fungi, prions, protozoa and algae, collectively known as 'microbes'. Biochemistry • • the branch of science that explores the chemical processes within and related to living organisms. a laboratory-based science that brings together biology and chemistry. Bioethics • • • • the study of the ethical issues emerging from advances in biology and medicine. It is also moral discernment as it relates to medical policy and practice. concerned with the ethical ques ons that arise in the relationships among life sciences, biotechnology, medicine, and medical ethics, politics, law, theology, and philosophy. includes the study of values relating to primary care and other branches of medicine ("the ethics of the ordinary"). Science • a process for learning about the natural world. Products: o Chemotherapy o Genetics o Treatment response Scientific Method • 1. 2. 3. 4. 5. 6. 7. a process used when conducting experiments and exploring observations. Ask a question o will include one of the key starters, which are how, what when, why, where, who or which. o should also be measurable and answerable through experimentation. o often something that can be measured with a numerical result, although behavioral results are part of the scientific method as well. Perform Research o conduct preliminary background research to prepare yourself for the experiment. Establish your hypothesis o A hypothesis is an educated guess that seeks to answer a question that can be systematically tested. Test your hypothesis by conducting an experiment o test your hypothesis by conducting an experiment. Make an observation o Assess your scientific process and make sure that the conditions remain the same throughout all testing measures. Analyze the results and draw a conclusion o You can now take your experiment findings and analyze them to determine if they support your hypothesis. Present the findings MICROBIOLOGY Classification Of Microorganism • Viruses • Bacteria • Archaea • Protozoa • Certain types of algae and fungi Microbes • Acellular Infectious Agents • • • o Phototropism is a plant’s response to stimuli (turning toward the light). Non-cellular objects that contaminate & affect living cells. PRIONS - An abnormal form of a normally harmless protein found in the brain that is responsible for a variety of fatal neurodegenerative diseases of animals, including humans, called transmissible spongiform encephalopathies. VIRUS - Infectious agents of small size and simple composition that can multiply only in animals, plants, or bacteria living cells. The name is from a Latin word meaning “slimy liquid” or “poison.” Viruses cannot reproduce outside a host cell and cannot metabolize on their own. Viruses often infect prokaryotic and eukaryotic cells causing diseases. Cellular Microorganism • • Characteristics of Life 1. Adaptation through evolution o All forms of life evolve adapt to the external environment, change their heritable traits, and prepare future generations for more efficient life processes. 2. Cellular organization o The general structures move along a line from cell to tissue to organ to being, which gives us the word “organism,” a living thing with organ systems. 3. Growth and development o Organisms reproduce with immature and small copies of themselves to conserve resources. 4. Heredity o Life transfers characteristics to offspring via deoxyribonucleic acid (DNA) and ribonucleic acid (RNA); these are the building blocks of life. 5. Homeostasis o Maintaining a stable internal environment. 6. Metabolism o Chemical reactions inside cells, tissues, organs, and living beings perform various actions that keep the organism alive. 7. Reproduction o Successful organisms reproduce. 8. Response to stimuli an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. organisms whose bodies are composed of cells. Cellular organisms are further categorized into unicellular and multicellular. o Unicellular Organisms – organisms that consist of one single cell. o Multicellular organisms – composed of many cells. Prokaryotes • • • A microscopic single-celled organism that has neither a distinct nucleus with a membrane nor other specialized organelles. ARCHAEA - prokaryotes with unique characteristics that differentiate them from other forms of life. o METHANOGENS - produce methane gas as a metabolic byproduct. o HALOPHILES - Thrive in high-salt environments. o THERMOPHILES - Adapted to extremely high-temperature environments. o ACIDOPHILES - Can survive and thrive in acidic environments. BACTERIA o prokaryotic organisms, lacking a true nucleus. o ESCHERICHIA COLI - commonly found in the intestines of humans and animals. o STAPHYLOCOCCUS AUREUS - Known for causing skin infections, pneumonia, and food poisoning, often found on the skin and in the nose of healthy individuals. o MYCOBACTERIUM TUBERCULOSIS responsible for tuberculosis, a serious o infectious disease that primarily affects the lungs. LACTOBACILLUS - group of beneficial bacteria found in the gut and used in the fermentation of foods like yogurt and pickles. Types of Viruses Eukaryotes • • • • An organism consisting of a cell or cells in which the genetic material is DNA in the form of chromosomes contained within a distinct nucleus. EUKARYOTES ALGAE o Photosynthetic organisms that lack true roots, stems, and leaves. They can be unicellular or multicellular, forming colonies, and are found in aqua c and terrestrial environments. Algae play as a producer, contributing oxygen production and serving as a food source. o Chlorella, Sargassum, Spirogyra, Caulerpa EUKARYOTES FUNGI o Fungi are a kingdom of usually multicellular eukaryotic organisms that are heterotrophs, meaning they cannot produce their own food. They play essential roles in nutrient cycling in ecosystems and can reproduce both sexually and asexually. o Agaricus Bisporus, Pleurotus Ostreatus, Saccharomyces, Ustilago Maydis, Rhizopus Stolonifer EUKARYOTIC: PROTOZOA o Protozoa are single-celled eukaryotic microorganisms that belong to the kingdom Protista. Plays essential roles in various ecosystems and can be found in aquatic and terrestrial habitats. o Amoeba, Plasmodium, Trypanosoma, Pramacium, Giardia Lamblia BACTERIA • • • microscopic living organisms that have only one cell. Harmful bacteria examples include: o Streptococcus: Bacteria that cause strep throat. o Staphylococcus: Bacteria that cause staph infections. o Escherichia coli: Bacteria that cause E. coli infections. Examples of Pathogenic Bacteria: o Aerococcus urinae: Bacteria in urine that cause urinary tract infections. o Chlamydia trachoma s: Bacteria that cause a sexually transmitted infection (STI) called chlamydia. o Bordetella pertussis: Bacteria that cause whooping cough. ANTIBIOTICS • Can treat most types of bacterial infections. 1. 2. 3. 4. 5. WATER WIND OBJECT FOOD ANIMALS VIRAL DISEASES OF HUMAN 1. INFLUENZA • caused by three different orthomyxoviruses: types A, B, and C. • best prevented with yearly vaccination, although new antiviral drugs can shorten the duration of symptoms. 2. SARS • represents a newly emerging viral disease caused by a coronavirus. • spread by person-to-person contact. • Include fever, headache, feeling of discomfort, and body aches. 3. HERPES SIMPLEX • a wide spectrum of viral diseases commonly found in the environment. • cause cold sores (HSV-1) and genital herpes (primarily HSV-2). • Neonatal herpes is a possible lifethreatening disease transmitted from a herpes simplex-infected mother during childbirth. 4. CHICKENPOX • Another member of the herpes viruses is varicella-zoster. • one of the most highly contagious diseases. • same virus causes shingles in adults, which can be a painfully debilitating disease. • Acyclovir has been successful at lessening the symptoms. Protozoa • VIRUS • • TRANSMISSION THROUGH DIFFERENT VECTORS Not living things. It needs host to be able to survive. • microscopic unicellular eukaryotes that have a relatively complex internal structure and carry out complex metabolic activities. often spread through contaminated water. • o Some protozoa are parasites, which means that they need to live on or in another organism (like an animal or plant) to survive. Different classifications of Protozoa 1. Mastigophora or Flagellated protozoans 2. Sarcodina or Amoeboids • 3. Sporozoa or Sporozoans 4. Ciliophora or Ciliated protozoans Immune System • A large network of organs, white blood cells, proteins, and chemicals. Immunity • • • Protection against harmful substances is what immunity does. a type of defense that protects the body from infections caused by microorganisms like bacteria, viruses, and other toxic agents. Its main purpose is to win over poten al threats to our bodies. Types of Immunity • Innate Immunity o triggered by small cuts and is a nonspecific defense mechanism. o It provides immediate protection against pathogens. Four (4) Components or Barriers of Innate Immunity: Physical or Anatomical Barriers They stop the entry of pathogens and foreign bodies into the body. Physiological Barriers - functional secretions that inhibit the growth of microorganisms that are capable of passing through physical barriers. Cellular or Phagocytic Barriers bactericidal or microbial barriers. They destroy these organisms by phagocytosis. Immune cells of the body. Inflammatory Barriers - Provide a barrier against infec on from spreading further outwards. Acquired Immunity o Involves immune cells that recognize and remember specific invaders, such as the immune system in the flu vaccine. o The acquired immune system is made up of: T cells (also called T lymphocytes) are made in bone marrow. B cells (also in the tissue between the body's cells). (B lymphocytes) are made in the bone marrow, where they mature into specialized immune system cells. Anti bodies in the blood and other bodily fluids. They travel around the body in the bloodstream. They are made by the immune system to fight germs and foreign substances. o Two (2) Types of Acquired Immunity: Active Immunity • Occurs when the body produces its immune response to a pathogen or an antigen. • Categorized in Two (2) Types: o Naturally Acquired Active Immunity - is acquired from exposure to the disease organism through infection with the actual disease. o Artificially Acquired Active Immunity - any immunization with an antigen. • Two (2) Distinct Forms of Acquired Active Immune Response: o Cell-mediated immunity/ response - Involves T cells directly attacking infected or abnormal cells. o Humoral immunity/ response - Produces antigen-specific antibodies and is primarily driven by B cells, which circulate in the blood and other bodily fluids, targe ng pathogens and toxins. Passive Immunity • Provided by antibodies or immune cells from another source, provides immediate but temporary protection. • Categorized in Two (2) Types: o Naturally Acquired Passive Immunity - Occurs during pregnancy, in which certain antibodies are passed from the maternal blood into the fetal bloodstream in the form of IgG. o Artificially Acquired Passive Immunity - Results when antibodies or lymphocytes produced outside the host are introduced into a host. Vaccination • • a medical intervention used to protect individuals from infectious diseases. involves the administration of a vaccine, which is a substance designed to stimulate the body's immune system to recognize and fight pathogens such as bacteria, viruses, or other microorganisms. HISTORY OF VACCINATION Early Practices: Variolation 1. Ancient China and India (10th Century) – Variolation: One of the earliest methods to prevent smallpox. Dried smallpox scabs were inhaled or scratched into the skin, inducing a mild form of the disease and providing immunity. – Practices Spread: This method spread to other parts of Asia, Africa, and the Middle East over the centuries. The Birth of Modern Vaccina on: Edward Jenner 2. Edward Jenner (1796) – Smallpox Vaccine: The English physician Edward Jenner is – often credited with developing the first true vaccine. He noticed that milkmaids who contracted cowpox, a milder disease, did not get smallpox. In 1796, Jenner inoculated a boy named James Phipps with cowpox pus and later exposed him to smallpox. The boy did not develop smallpox, demonstrating immunity. – "Vaccination": The term comes from “vacca,” the Latin word for cow, in honor of Jenner's work with cowpox. 19th Century: Expansion and Development 3. Louis Pasteur (1880s) – Rabies Vaccine: Louis Pasteur, a French microbiologist, developed the first laboratory-created vaccine for rabies by attenuating (weakening) the virus. His successful use of the vaccine on a boy bitten by a rabid dog in 1885 was a landmark event. – Cholera and Anthrax Vaccines: Pasteur also developed vaccines for chicken cholera and anthrax. Early 20th Century: Vaccines for Major Diseases 4. Development of Vaccines for Diphtheria, Tetanus, and Pertussis (DTP) – 1920s-1940s: Vaccines for diphtheria, tetanus, and pertussis (whooping cough) were developed and became widely used. – Combination Vaccine (1940s): The DTP combined vaccine was introduced, protecting against all three diseases in one shot. 5. Polio Vaccine – Jonas Salk (1955): Developed the first effective polio vaccine using inactivated (killed) virus. It was administered by injection. – Albert Sabin (1961): Developed an oral polio vaccine using a live attenuated virus, which became widely used due to its ease of administration and strong immunity. Late 20th Century: Advances and Eradication 6. Measles, Mumps, and Rubella (MMR) Vaccine (1971) – Combined Vaccine: The MMR vaccine combined vaccines for measles, mumps, and rubella into a single shot, making vaccina on more convenient and widespread. 7. Eradication of Smallpox (1980) – Global Effort: The World Health Organization (WHO) led a global vaccina on campaign that resulted in the eradication of smallpox, declared in 1980, marking a significant triumph in public health. 21st Century: Modern Vaccines and Technology 8. Human Papillomavirus (HPV) Vaccine (2006) – Cervical Cancer Prevention: The HPV vaccine was introduced to prevent cervical and other cancers caused by human papillomavirus. 9. mRNA Vaccines (2020) – COVID-19 Vaccines: The COVID-19 pandemic accelerated the development and deployment of mRNA vaccines by PfizerBioNTech and Moderna. Ongoing Developments 10. Research and Innovation – New Vaccines: Continued research is leading to new vaccines for diseases such as malaria, HIV, and various cancers. – Improved Delivery Methods: Advances in vaccine delivery methods, including nasal sprays, patches, and oral vaccines, aim to make vaccina on more accessible and effective. How Vaccines Work 1. Introduction of Antigens 2. Immune System Activation 3. Memory Cell Formation Types of Vaccines 1. Inactivated (Killed) Vaccines 2. Live Attenuated Vaccines 3. Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines 4. mRNA Vaccines 5. Vector Vaccines Benefits of Vaccination 1. 2. 3. 4. Prevention of Disease Herd Immunity Reduction of Disease Severity Eradication of Diseases Safety and Efficacy – Clinical Trials: Vaccines undergo rigorous testing in multiple phases of clinical trials to ensure they are safe and effective. – Monitoring: After approval, vaccines continue to be monitored for safety and effectiveness through surveillance systems and studies. Common Misconceptions 1. Vaccines Cause Severe Side Effects 2. Vaccines Cause the Disease They Are Meant to Prevent 3. Natural Immunity is Better BIOCHEMISTRY – study of the chemical processes in living organisms. – Deals with the structure and function of cellular components such as carbohydrates, lipids, proteins, and nucleic acid. – Become the foundation for understanding all biological processes. – Seeks to unravel the complex chemical reactions. Biochemistry Involves the Study of: 1. Chemical constituents of living matter. 2. Chemical changes occur in the organism during digestion, absorption, and excretion. 3. Chemical changes occur during the growth and multiplication of the organism. 4. Transformation of one form of chemical constituent to the other. 5. Energy changes are involved in such transformation. MACROMOLECULES (Biomolecules) – a very large molecule important to biological processes, such as a protein or nucleic acid. – composed of thousands of covalently bonded atoms. – Monomers - Many macromolecules are polymers of smaller molecules (include Carbohydrates, Lipids, Proteins, and Nucleic acids) Micromolecules (Acid Soluble) • The acid soluble pool contains chemicals with a small molecular mass of 18 to 800 Daltons approximately (include sugars, amino acids, water, minerals, and nucleotides) Carbohydrates – – “Hydrates of carbon" biological molecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms. – Functions: o Provides energy. o Stored energy. o Water insoluble carbohydrates are major components of the cell wall in plant cells and cell membrane in animal cells. o lubricants in skeletal joints and help the cells to stick with each other and remain in place. o Fibers promotes good digestive health by reducing constipation and lowering the risk of digestive tract diseases. – also referred to as sugars or saccharides (derived from the Greek sákkharon, meaning “sugar”) – SUGAR o a type of carbohydrate, which is a biomolecule consisting of carbon (C), hydrogen (H), and oxygen (O) atoms. o categorized based on the number of saccharide units they contain. Classes of Carbohydrates • Monosaccharide o simplest form of sugar, consisting of a single saccharide unit. (include glucose, fructose, and galactose) o Monosaccharides are the building blocks of more complex sugars. • Disaccharide o Composed of two monosaccharide units linked together by a glycosidic bond. o Examples include sucrose (table sugar, made of glucose and fructose), lactose (milk sugar, made of glucose and galactose), and maltose (malt sugar, made of two glucose units). • Oligo (a few) saccharide o any carbohydrate of from three to six units of simple sugars (monosaccharides). o Most of the few naturally occurring oligosaccharides are found in plants. • Poly(many)saccharide o sugar polymers containing more than 20 or so monosaccharide units, and some have hundreds or thousands of units. Proteins • large, complex molecules essential for body function. • Made up of amino acids in long chains. • consist of 20 different amino acids. • Sequence of amino acids determines protein structure and function. • DNA nucleotides code for amino acids. The Four Levels of Protein Structure • Primary Structure o Unique sequence of amino acids in a polypeptide chain. o Determined by the gene's nucleotide sequence. o For example, Insulin has two polypeptide chains, A and B. • Secondary Structure o A Regular structure formed by interactions between nearby amino acids. o Includes α-helices and β-pleated sheets. o Stabilized by hydrogen bonds. • Tertiary Structure o An Overall 3D shape of the polypeptide. o Formed by interactions among R groups (polar, nonpolar, acidic, basic). o Hydrophobic R groups inside, hydrophilic R groups outside. o Disulfide linkages between cysteine residues. • Quaternary Structure • Arrangement of multiple polypeptide chains. • Stabilized by weak interactions and sometimes enzymes. • Only applies to multi-subunit proteins. Protein Folding • Unique amino acid sequence dictates protein shape. • Shape determines protein function. Changing the Shape of a Protein (Denaturation) • Temperature, pH, and chemicals can alter protein shape. • Denatured proteins lose function. • For example, Pepsin works at low pH in the stomach. Reversing Denaturation • Possible if primary structure is intact. • For the same example, Pepsin in the stomach. • Extreme cases like cooking can cause irreversible denaturation. Enzymes and Homeostasis • Enzymes catalyze biochemical reactions within narrow temperature and pH ranges. • Homeostatic mechanisms maintain optimal conditions for enzyme function. Chaperone Proteins • Chaperonins assist in protein folding, • Prevent aggregation of polypeptide chains, and • Disassociate once the protein is correctly folded. AMINO ACIDS • serve as the fundamental components of proteins • The body utilizes amino acids to synthesize proteins. • play numerous crucial roles in your body. Amino acids help. AMINO ACID GROUPS 1. Hydrophobic or Nonpolar Groups: These side chains do not interact well with water. 2. Hydrophilic or Polar Groups: These side chains interact well with water. 3. Acidic or Negatively Charged Groups: These side chains have a negative charge. 4. Basic or Positively Charged Groups: These side chains have a positive charge. Lipids • a diverse group of organic compounds that are essential components of living cells. • characterized by their insolubility in water but solubility in nonpolar solvents like chloroform or ether. • Functions: o Energy storage o Cellular structure o Hormone Production o Insulation o Signaling Molecules o Vitamin Absorption Types of Lipids • Triglycerides o Composed of glycerol and three fatty acids. o The fatty acids are attached to the glycerol through ester bonds. • Phospholipids o have a glycerol backbone, two fatty acids, a phosphate group, and polar head group. o have a hydrophilic head and hydrophobic tail. • Steroids o Steroids are lipids with a structure consisting of four-fused hydrocarbon rings (steroidal backbone). • Waxes o are esters of long chain fatty acids and long chain alcohols. Fatty Acids • composed of carbon, hydrogen, and oxygen • arranged as a variable-length linear carbon chain skeleton with an even number of atoms at one end. • with 2 to 30 carbon atoms or more, but the most common and important ones have 12 to 22 carbon atoms • found in many different animal and plant fats • provides energy • serve as building blocks for various lipid molecules • building blocks of fat in our bodies and the food we consume • joined in groups of three to form a molecule known as a “triglyceride” Classification of Fatty Acids • classified into three types based on their degree of saturation/unsaturation in the carbon chain: o If there is no double bond, the fatty acid is saturated. o If there is one double bond, the fatty acid is monounsaturated, o If there are two or more double bonds, the fatty acid is polyunsaturated. Glycerol (C3H8O3) • also known as glycerin • non-toxic, viscous, colourless, and simple triol compound • mostly used in dermatological treatments and in the food industry as a preservative and sweetener • has 3 carbon atoms, 8 hydrogen atoms, and 3 oxygen atoms • soluble in water and is hygroscopic • its backbone is found in all lipids known as • “triglycerides” • Its use ranges from pharmaceuticals to the food industry and in cosmetics (like soaps) NUCLEIC ACID • biomolecules that contain the blueprints for making proteins. Nucleic acids also transmit genetic info to the next generation. • organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. • They serve as monomeric units of the nucleic acid polymers. • it contains “CHONP” • o carbon o hydrogen o oxygen nitrogen o phosphorus Metabolic Pathway • It is a biopolymer made up of monomers called nucleotides. o Monomer: Nucleotides o Polymer: Nucleic Acid o Examples: DNA, RNA TWO TYPES OF NUCLEIC ACID DNA (deoxyribonucleic acid) DNA replicates and stores genetic information. FUNCTIONS STRUCTURE It is the blueprint for all genetic information contained within an organism. • • RNA (ribonucleic acid) RNA converts the genetic information contained within DNA to a format used to build proteins. It consists of two strands, arranged in a double helix made up of subunits called nucleotides. It has one strand and is also made up of nucleotides. Much longer polymer than RNA. It is variable in length but much shorter than long DNA polymers. LENGTH Several cm in length if unraveled. SUGAR • • Enzymes • Deoxyribose Ribose DNA is found in nucleus, with small amount of DNA also present in mitochondria. RNA forms in the nucleolus, then moves to specialized regions of cytoplasm. Adenine, Thymine, Cytosine and Guanine Adenine, Uracil, Cytosine and Guanine Series of interactions between molecules in a cell that lead to assembly of new molecules in a cell, turn genes on and off, maintain and control the flow of information, energy and biochemical compounds are called biological pathways. Enzymes control metabolic pathways. The enzymes change the substrate at each step in the metabolic pathway in order to get the final product at the end. Metabolic pathways can be reversible or irreversible. Almost all pathways are reversible. CATABOLIC PATHWAY o Catabolic reactions involve the breaking of bonds; whenever chemical bonds are broken, energy is released. o This type of pathway releases energy and is used to break down large molecules into smaller ones (degradation). o example of a catabolic reaction is the process of food digestion ANABOLIC PATHWAY o Anabolic reactions involve the creation of bonds, which require energy. o This type of pathway requires energy and is used to build up large molecules from smaller ones (biosynthesis). o example of an anabolic reaction is photosynthesis. • vital proteins involved in metabolic pathways. Some enzymes can be found embedded within the cell membrane. Other proteins found embedded within the membrane act as pumps (e.g. the sodium potassium pump that pumps sodium out of the cell and potassium into the cell) and pores (which allow ions of a particular size to pass through the membrane and pores) How is sugar converted into energy LOCATION BASE • The conversion of sugar into energy primarily involves a series of metabolic pathways that break down glucose, a simple sugar, to produce ATP (adenosine triphosphate), the energy currency of the cell. EXAMPLE OF METABOLIC PATHWAYS Cellular Respiration • AEROBIC RESPIRATION o Complete catabolism of glucose. o • Occurs in three phases, each of which is a biochemical pathway: Glycolysis (Anaerobic) • “Glycolytic Pathway” • Nine-step biochemical pathway involving nine separate biochemical reactions, each of which requires a specific enzyme. • Can take place either in the presence of oxygen or in the absence of oxygen. Produces very little energy ---- a net yield of only two molecules of ATP. • Takes place in the cytoplasm of both prokaryotic and eukaryotic cells. Krebs cycle • The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetylCoA derived from carbohydrates, fats, and proteins. The Electron Transportation • Also called the “electron transport system” or “respiratory chain.” • Consists of a series of oxidationreduction reactions. • Many different enzymes are involved in the electron transport chain, including cytochrome oxidase. o Glucose + Oxygen = Carbon dioxide + Water + Energy ANAEROBIC RESPIRATION o Glucose → Alcohol + Carbon dioxide + Energy o Glucose → Lactic acid + Energy o Fermentation Alcoholic Fermentation • The process by which glucose and fructose, two sugars, are converted anaerobically into carbon dioxide and ethanol. Lactic Acid Fermentation • A form of anaerobic respiration that occurs in yogurt bacteria (Lactobacillus and other species). It is also produced during extreme exercise. o Oxidation-Reduction (Redox) Reactions A redox reaction, also known as an oxidation-reduction reaction, is a chemical reaction in which electrons are transferred between chemical species, such as atoms, ions, or molecules. o Biosynthesis of Organic Compounds o o Energy is required to produce organic compounds. This process can occur through photosynthesis, which uses light energy for biosynthesis, or through chemosynthesis, which uses chemical energy for biosynthesis. Photosynthesis The process by which green plants and some other organisms convert light energy into chemical energy is known as photosynthesis. In green plants, light energy is absorbed during photosynthesis and used to transform water, carbon dioxide, and minerals into organic compounds that are high in energy and oxygen. Chemosynthesis The process by which bacteria or other organisms use chemicals as an energy source to produce food, typically in the absence of sunlight. Minerals • In biology, minerals refer to essential nutrients that organisms need in small amounts to maintain health and perform vital functions. These minerals include elements like calcium, iron, and zinc, which are important for bone strength, oxygen transport, and enzyme activity in the body. TYPE OF ESSENTIAL MINERALS • Macrominerals (macroelements) o Macro minerals are essential nutrients required by the body in relatively larger amounts to maintain proper health and function. o K, Na, Cl, Ca, P, Mg, S • Microminerals (microelements) “trace elements" o Micro minerals are essential nutrients needed by the body in trace amounts for various physiological functions. o Fe, Zn, I, Se, Cu, Mn, F, Cr, Mo • The body tightly regulates mineral levels through hormonal control, storage in tissues, and excretion to ensure balance and proper physiological functioning. Mineral Deficiencies • Minerals are crucial for bodily functions, and insufficient intake or absorption can lead to significant health problems such as iron-deficiency anemia and calcium-deficiency osteoporosis. IMPORTANCE OF SOLUBILITY • FUNCTIONS OR ROLES IN METABOLIC PATHWAYS • • • • Energy Metabolism o Energy metabolism refers to the processes by which cells convert nutrients into energy that can be used to perform various biological functions. (Example: Essential minerals support ATP synthesis and electron transport chain for cellular energy.) Enzyme Activation o Enzyme activation refers to the process of preparing enzymes to catalyze chemical reactions by altering their structure or environment so they can perform their specific roles effectively. (Example: Minerals are enzyme cofactors.) Structural Support o Structural support refers to the role of substances or materials that provide strength, stability, and form to biological structures such as bones, tissues, or cell membranes. (Example: Bone & teeth support, protein/enzyme structure) Electrolyte Balance o Electrolyte balance refers to the maintenance of proper levels of ions such as sodium, potassium, calcium, and magnesium in bodily fluids, crucial for various physiological functions including nerve signaling and muscle contraction. (Example: Cellular Balance) MINERAL ABSORPTION, REGULATIONS, AND DEFICIENCIES Mineral Absorption • Mineral absorption involves the uptake of minerals from food, influenced by dietary intake, bioavailability, and individual health. Mineral Regulation The solubility of minerals determines their bioavailability, meaning how readily they can be absorbed by living organisms. Acid-soluble minerals generally dissolve more easily in water or digestive juices, making the contained mineral ions more readily available for uptake and utilization in metabolic pathways.