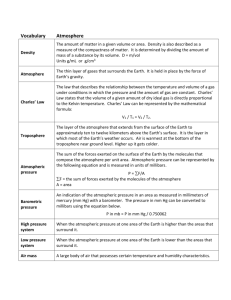
LENOVO UNIT- 6 DENSITY AND PRESSURE Mass(m) The mass of a body is a measure of the amount of substance in that body. It is a scalar quantity. Its SI unit is kilogram (kg). It is measure with a balance. Volume(V) The quantity of space an object takes up is called its volume. The SI unit of volume is cubic metre (𝒎𝟑 ). 1 cubic metre (𝑚3 ) is the volume of a cube measuring (1m × 1m ×1m). Other units are Litre (L) and cubic centimeter( 𝑐𝑚3 ). 1 litre is the same as 1 cubic decimetre (𝑑𝑚3 ). 1 litre = 1 𝑑𝑚3 =1000 𝑐𝑚3 =1000 millilitres (ml) 1 cubic centimeter (𝑐𝑚3 ) is the same as 1 millilitres., Measuring volume The volume of Liquid – using measuring cylinder (Pour liquid into cylinder, the level on the scale gives the volume) Regular solid – using geometrical equation Irregular solid – using a displacement can and a measuring cylinder Measuring cylinder displacement can 1 LENOVO When making a reading with measuring cylinder, the eyes must be level with the bottom of the curved liquid surface, the meniscus. The meniscus formed by mercury is curved oppositely to that of other liquids and the top is read. Density (ρ) The density of a substance is defined as its mass per unit volume. In symbol, where, Density (ρ) = 𝑚𝑎𝑠𝑠(𝑚) 𝑣𝑜𝑙𝑢𝑚𝑒(𝑣) m=mass(kg) V=volume (𝑚3 ) Ρ=density(kg/𝑚3 ) Density is a scalar quantity. Its SI unit is kilogram per meter cube (kg/𝒎𝟑 ). If mass (g) and volume (𝑐𝑚3 ), then its density is gram per cubic centimeter(g/𝑐𝑚3 ) The density of the liquids does not vary with pressure but the density of gases varies considerably with pressure (gases are readily compressible; liquids are not). An object will float in a liquid if its density is less than the density of the liquid. When we compare the density of the objects, we are actually comparing objects with the same volume but have different masses. A denser object is one that has the same volume but greater mass. Pressure(P) Pressure is defined as the force acting on per unit area. In symbol, where, 𝑭 P=𝑨 P= pressure (Pa) F=applied force(N) A= area (𝑚2 ) Pressure is a scalar quantity. Its SI unit is pascal (Pa) or newton per meter squared (N 𝒎−𝟐 ). A sharp knife cuts more easily than a blunt knife because with a sharp knife all the force is concentrated into a much smaller area. 2 LENOVO In hospital, bed sores are a problem for patients confined to bed for long periods of time. The skin in contact with the bed is not as tough as the skin under your feet. As a result, the skin in the contact area is easily rubbed away, causing bed sores. Increasing the pressure by reducing the area The studs on a football boot have only a small area of contact with the ground. The area under the edge of a knife`s blade is extremely small ( to push easily through the material) Under the tiny area of the point of a drawing pin, the pressure is far too high for the wood to withstand Reducing the pressure by increasing the area Skis have a large area to reduce the pressure on the snow Wall foundations have a large horizontal area A load-spreading washer ensures that the nut is not pulled into the wood ATMOSPHERIC PRESSURE (𝑷𝒂𝒕𝒎 ) The pressure exerted by the atmosphere on all living and non-living thing on earth is called atmospheric pressure. The earth is surrounded by the atmosphere (which consists of masses of gases has weight ) up to a height of many (many) miles. The weight of the Earth`s atmosphere pushing down on each unit area of the earth`s surface constitutes the atmospheric pressure. The magnitude of atmospheric pressure at the earth`s surface is about 100 kN𝑚−2. The atmospheric pressure does vary from day to day and place to place. Its value drops as we ascend above sea level and increases as descend below sea level. At high altitudes where the air pressure is lower, breathing is difficult and nose bleeding may occur. Bleeding results from the pressure difference between the body and the external pressure. STANDARD ATMOSPHERE The standard atmosphere is the mean atmospheric pressure naturally existing at sea level on the surface of the earth. At sea level, air molecules exert a pressure of 1.013× 105 Pa. It is equivalent to the pressure exerted by a column of mercury 760 mm high. It is also called one atmosphere. This is equivalent to placing a 10 N weight (mass of 1kg) over an area of 1 𝑐𝑚2 (0.001𝑚2 ) PRESSURE IN LIQUID (𝑷𝒍𝒊𝒒) A liquid exerts pressure because of its weight. This causes pressure on the container, and pressure on any object in the liquid. The pressure in a liquid depends on the height of liquid column (depth of liquid) and the density of the liquid. 3 LENOVO Calculating the pressure in a liquid The container has a base area A and it is filled to a depth h with a liquid of density ρ (rho). Volume of liquid =base area ×depth = Ah Mass of liquid = density ×volume = ρAh Weight of liquid = mass ×g = ρAhg (g= 10 N/kg) Force on base = weight of liquid = ρAhg This force is acting on an area A. 𝑓𝑜𝑟𝑐𝑒 Pressure = 𝑎𝑟𝑒𝑎 = 𝜌𝐴ℎ𝑔 = ρgh 𝐴 Pressure of liquid = 𝑷𝒍𝒊𝒒 = ρgh Total pressure of liquid at the depth of h, atmosphere total pressure= atmospheric pressure + liquid pressure liquid In real case, the pressure at any depth in a liquid is the sum of the atmospheric pressure and the pressure due to the height of the liquid column. 4 LENOVO 𝐏𝐭𝐨𝐭𝐚𝐥 = 𝐏𝐚𝐭𝐦 + 𝐏𝐥𝐢𝐪𝐮𝐢𝐝 Aqualung divers do not normally go beyond a depth of 30 m because the pressure of the water becomes too great for the human body to withstand. PROPERTIES OF LIQUID PRESSURE The pressure exerted by the liquid has its own properties. (1) Pressure at one depth acts equally in all directions (the liquid pushes on every surface in contact with it, no matter which way the surface is facing) (2) Pressure increases with depth (the deeper into a liquid, the greater the weight of liquid above and the higher the pressure) (3) All points at the same depth in a liquid are at the same pressure. (4) Pressure does not depend upon the shape or base area of container. (5) Pressure can be transmitted throughout the liquid. (6) Pressure depends on the density of the liquid. (the more dense the liquid, the higher the pressure at any particular depth) 5 LENOVO PRESSURE DEVICES AND MACHINES The mercury Barometer A barometer is an instrument used for measuring atmospheric pressure. A mercury barometer consists of a thick-walled glass tube sealed at one side and is about one meter in length. The glass tube filled with mercury is inverted with its opening end dipped into a mercury reservoir. The atmosphere pushes against the mercury bath, which in turn pushes the mercury up the tube. The tip of the glass tube is a vacuum space that allows mercury level in the tube to raise and drop. The vertical height of the mercury column (h) gives the required atmospheric pressure. At sea level, the barometer will show the reading of 760mm (0.76 m) The space at the top of the barometer tube is a vacuum called Torricellian vacuum and exert no pressure (except for a little mercury vapour). If mercury is replaced by water, the vertical column of water equivalent to the atmospheric pressure is approximately 10 m. The height of the mercury column remains the same even when we tilt the column. Manometer A manometer is a device used to measure the gas pressure. It consists of a U-tube filled with a column of liquid (mercury, water or oil) When the manometer is not connected to a gas supply, the liquid levels are equal because atmospheric pressure acts on both surfaces of the liquid. 6 LENOVO When one end of the tube is connected to a gas supply, the pressure exerted by the gas changes the levels of the liquid. Fig (1) fig(2) fig(3) For fig (2), 𝑃𝑔𝑎𝑠 ˃ 𝑃𝑎𝑡𝑚 , 𝑃𝑔𝑎𝑠 = 𝑃𝑎𝑡𝑚 + ρgh (h=the difference in height of the liquid column) For fig (3), 𝑃𝑔𝑎𝑠 ˂ 𝑃𝑎𝑡𝑚 , 𝑃𝑔𝑎𝑠 = 𝑃𝑎𝑡𝑚 - ρgh Hydraulic press A hydraulic press is a machine in which the force is transmitted by liquid under pressure. They make use of these properties of liquids; Liquid are virtually incompressible (i.e. they cannot be squashed) 7 LENOVO If a trap liquid is put under pressure, the pressure is transmitted equally to all parts of the liquid. The pressure produced by the effort is transmitted through the fluid (usually oil) and it exerts the same pressure on the large piston. For a hydraulic press, 𝐏𝐢𝐧 = 𝐏𝐨𝐮𝐭 𝑭𝒊𝒏 𝑭𝒐𝒖𝒕 = 𝑨𝒊𝒏 𝑨𝒐𝒖𝒕 A hydraulic system is used to lift heavy loads like a car. A small force is applied at a small piston and the heavy load to be lifted is placed on the large piston. FLOATING AND SINKING Upthrust Upthrust is the upward push of the surround liquid on an object immersed in it. Any object in a liquid, whether floating or submerged, is acted on by upward force or upthrust. This makes it seem to weigh less than it does in air. An upthrust force also acts on objects by the atmosphere (such as helium balloon). Floating and sinking When a body is placed in a liquid, there may be three possibilities. (1) If the weight of the body is greater than the upthrust, the body will sink. (2) If the weight is smaller, the body will rise up to the surface. (3) A body will float in a liquid if the upthrust of liquid is equal to its weight. There are two factors that decide whether an object can float or sink in a liquid. For an object can float or sink in a liquid, The object must be less dense than the liquid. The shape of the object must enable it to float. Archimedes’ principle When a body is wholly or partly submerged in a fluid, the upthrust equals the weight of fluid displaced. 8 LENOVO Initial weight – Final weight = upthrust = weight of liquid displaced = 𝝆𝒍𝒊𝒒Vg Principle of floatation A floating body displaces its own weight of fluid. If a material floats, its density must be less than (or equal to ) that of the fluid supporting it. An object will float if the upthrust on it is enough to support its weight. The weight of fluid it displaced is equal to its own weight. A floating ship displaces a weight of water equal to its weight including that of the cargo. The load lines (called the Plimsoll mark) on the side of a ship show how long in the water it can lie and still be safely and legally loaded under different conditions. A submarine sinks by taking water into its buoyancy tanks. Once submerged, the upthrust is unchanged but the weight of the submarine increases with the inflow of water and it sinks faster. To surface, compressed air is used to blow the water out of the tank. A hot air balloon will rise upwards if its total weight is less than the weight of the air it displaces. Archimedes’ principle is true not only for the liquid but also for the gases. 9 LENOVO 10