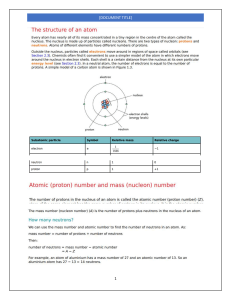
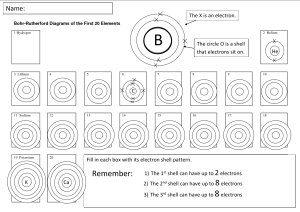
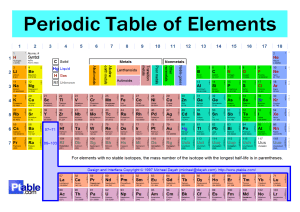
Head to savemyexams.com for more awesome resources IGCSE Chemistry Edexcel 1.4 The Periodic Table Contents 1.4.1 Periodic Table: Basics 1.4.2 Electronic Configurations 1.4.3 Classify Metals & Non-Metals 1.4.4 Electronic Configuration & Reactivity Page 1 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Your notes Head to savemyexams.com for more awesome resources 1.4.1 Periodic Table: Basics Your notes Arrangement of the Periodic Table There are over 100 chemical elements which have been isolated and identified Elements are arranged on the periodic table in order of increasing atomic number Each element has one proton more than the element preceding it This is done so that elements end up in columns with other elements which have similar properties The table is arranged in vertical columns called groups and in rows called periods Period: These are the horiz ontal rows that show the number of shells of electrons an atom has and are numbered from 1 - 7 E.g. elements in period 2 have two electron shells, elements in period 3 have three electron shells Group: These are the vertical columns that show how many outer electrons each atom has and are numbered from 1 – 7, with a final group called group 0 (instead of group 8) E.g. group 4 elements have atoms with 4 electrons in the outermost shell, group 6 elements have atoms with 6 electrons in the outermost shell and so on The Periodic Table Page 2 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Your notes The Periodic Table of the Elements Exam Tip The atomic number is unique to each element and could be considered as an element's “fingerprint”. The number of electrons changes during chemical reactions, but the atomic number does not change. Page 3 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources 1.4.2 Electronic Configurations Your notes Deducing Electronic Configurations We can represent the electronic structure of atoms using electron shell diagrams Electrons orbit the nucleus in shells and each shell has a different amount of energy associated with it The further away from the nucleus, the more energy a shell has Electrons first occupy the shell closest to the nucleus which can hold a maximum of 2 electrons When a shell becomes full of electrons, additional electrons have to be added to the next shell The second shell and third shell can hold 8 electrons each The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons In most atoms, the outermost shell is not full and therefore these atoms react with other atoms in order to achieve a full outer shell of electrons (which would make them more stable) In some cases, atoms lose electrons to entirely empty this shell so that the next shell below becomes a (full) outer shell Deducing electron configuration You should be able to write the electron configuration for the first twenty elements Electronic Configuration of the First 20 Elements Table Page 4 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Your notes Page 5 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Your notes Note: Although the third shell can hold up to 18 electrons, the filling of the shells follows a more complicated pattern after potassium and calcium. For these two elements, the third shell holds 8 and the remaining electrons (for reasons of stability) occupy the fourth shell first before filling the third shell Exam Tip You should be able to represent the first 20 elements using either electron shell diagrams or written electronic configuration. Page 6 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Electronic Configurations & the Periodic Table Electronic configuration: The arrangement of electrons into shells for an atom (e.g. electronic configuration of carbon is 2 . 4) Electronic configuration and position in periodic table The number of notations in the electronic configuration will show the number of shells of electrons the atom has, showing the period The last notation shows the number of outer electrons the atom has, showing the group Example: Electronic configuration of chlorine: Shorthand electronic configuration Period: The red numbers below the electronic configuration show the number of notations which is 3, showing that a chlorine atom has 3 shells of electrons Group: The last notation, which is 7, shows that a chlorine atom has 7 outer electrons matching group 7 of the periodic table The position of chlorine on the periodic table Page 7 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Your notes Head to savemyexams.com for more awesome resources Your notes The position of chlorine in the periodic table Exam Tip All of the shells up to the outer shell will be full. Electron transfer occurs with electrons from the outer shell only.You can use the term ‘shell’ or ‘energy level’ to describe the space that electrons occupy. Page 8 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources 1.4.3 Classify Metals & Non-Metals Your notes Classify Metals & Non-Metals We can use properties such as electrical conductivity and acid-base character to classify elements as metals or non-metals Characteristic Properties of Metals and Non-Metals Page 9 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Metals & Non-Metals in the Periodic Table The location of the metals and non-metals shows a clear pattern when highlighted on a periodic table Another thing that is striking, is that you can see that the vast majority of elements are metals A zig-zag line between the blue and purple elements in this diagram separates the metals on the left, from the non-metals on the right. Elements which border the line are hard to classify as they have characteristics of both sides, so the term semi-metal or metalloid is used. Page 10 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Your notes Head to savemyexams.com for more awesome resources 1.4.4 Electronic Configuration & Reactivity Similar Chemical Properties Chemical properties of elements in the same group Elements in the same group in the periodic table will have similar chemical properties This is because they have the same number of outer electrons so will react and bond similarly The group number of an element which is given on the periodic table indicates the number of electrons in the outer shell (valence electrons) This rule holds true for all elements except helium; although is in group 0, it has only one shell, the first and innermost shell, which holds only 2 electrons We can use the group number to predict how elements will react as the number of valence shell electrons in an element influences how the element reacts. Therefore, elements in the same group react similarly By observing the reaction of one element from a group, you can predict how the other elements in that group will react By reacting two or more elements from the same group and observing what happens in those reactions you can make predictions about reactivity and establish trends in reactivity in that group For example, lithium, sodium and potassium are in group 1 and can all react with elements in group 7 to form an ionic compound The group 1 metals become more reactive as you move down the group while the group 7 metals show a decrease in reactivity moving down the group Page 11 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Your notes Head to savemyexams.com for more awesome resources Why Noble Gases are Unreactive The elements in group 0 of the periodic table are called the noble gases They are all non-metal, monatomic (exist as single atoms), colourless, non-flammable gases at room temperature The group 0 elements all have full outer shells of electrons; this electronic configuration is extremely stable Elements participate in reactions to complete their outer shells by losing, gaining, or sharing electrons The Group 0 elements do not need to do this, because of their full outer shells which makes them unreactive and inert Other than helium which has 2 electrons in its outer shell, the noble gases have eight valence electrons (which is why you may see this group labelled “group 8”) Electronic configurations of the Noble gases: He = 2 Ne = 2, 8 Ar = 2, 8, 8 Kr = 2, 8, 18, 8 Xe = 2, 8, 18, 18, 8 The periodic table highlighting the noble gases – they occupy the group furthest to the right Page 12 of 12 © 2015-2024 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Your notes