BaTiO3 Ceramics: Dielectric & Ferroelectric Properties Study
advertisement

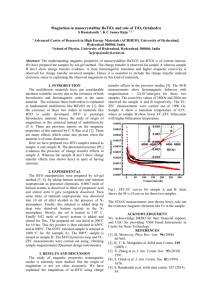
Applied Physics A (2018) 124:713 https://doi.org/10.1007/s00339-018-2126-z A comparative study of dielectric and ferroelectric properties of sol–gel-derived ­BaTiO3 bulk ceramics with fine and coarse grains Gasidit Panomsuwan1 · Hathaikarn Manuspiya2 Received: 17 June 2018 / Accepted: 19 September 2018 / Published online: 24 September 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Fine and coarse-grained B ­ aTiO3 (BTO) ceramics were prepared by conventional sintering of sol–gel-derived BTO nanopowders at 1150 and 1350 °C, respectively. Based on characterization results, fine and coarse-grained BTO ceramics had an average grain size of about 1 and 12 µm, respectively. They exhibited a tetragonal structure with tetragonality (c/a ratio) of 1.0105. The dielectric properties of fine and coarse-grained BTO ceramics were measured in the frequency range of 100 Hz–10 MHz and temperature range of −45–180 °C. A dominant dielectric relaxation was observed at high frequency above 1 MHz for both BTO ceramics. Room-temperature dielectric constant of fine-grained BTO (1502) was greater than that of coarse-grained BTO (1082) at 1 kHz due to the grain size effect. For temperature dependence measurement, dielectric constant of fine-grained BTO was less sensitive with changing temperature at phase transition than coarse-grained BTO. Polarization–electric field (P–E) loop of coarse-grained BTO at room temperature revealed a well-defined hysteresis loop, confirming its ferroelectric switching behavior. In contrast, a lossy hysteresis loop was found for fine-grained BTO owing to its high leakage current. Our results in this work provide a useful information and progress in the dielectric and ferroelectric properties of sol–gel-derived BTO bulk ceramics. 1 Introduction Barium titanate ­(BaTiO3, BTO) ceramic, a well-known perovskite oxide material, has long been considered as one of the most promising ferroelectric materials for several decades since the first discovery in 1941 [1, 2]. Currently, they are practically embedded in a broad range of miniature and integrated electronic devices, such as actuators [3], sensors [4], micro-electromechanical systems (MEMS) [5], and nonvolatile memories [6]. To date, a significant progress has Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00339-018-2126-z) contains supplementary material, which is available to authorized users. * Gasidit Panomsuwan gasidit.p@ku.ac.th * Hathaikarn Manuspiya hathaikarn.m@chula.ac.th 1 Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, Thailand 2 The Petroleum and Petrochemical College, Center of Excellence on Petrochemical and Materials Technology, Chulalongkorn University, Bangkok 10330, Thailand been made in optimizing and controlling dielectric and ferroelectric properties of BTO ceramics to realize the fabrication of advanced electronic devices with high performance and reliability. In BTO ceramics, grain size plays a significant role in determining their dielectric and ferroelectric properties [7, 8]. The dielectric constant of the BTO ceramics could reach the maximum value at grain sizes range of 0.8–1.3 µm [8–14]. A larger or smaller grain size than such a critical range results in the reduction of dielectric constant. The maximum dielectric constant of the BTO ceramics at an intermediate grain size can be attributable to several factors based on the effects of internal residual stress in each individual grain [9], 90° ferroelectric domain [11], and domain size [12]. Despite numerous efforts toward this direction, a deeper and wider understanding of the grain-size effects on the dielectric and ferroelectric properties of the BTO ceramics is now still considered to be a major research topic from both the scientific and technological viewpoints. More importantly, the grain size in the BTO ceramics is strongly associated with several experimental factors involving the preparation of raw BTO powders and ceramics (e.g., synthesis route, starting precursors, calcination and sintering temperatures) 13 Vol.:(0123456789) Content courtesy of Springer Nature, terms of use apply. Rights reserved. 713 Page 2 of 8 G. Panomsuwan, H. Manuspiya [8, 15–18]. Therefore, there are plenty of rooms remaining for further investigation and development of BTO ceramics. According to several earlier reports, nanocrystalline BTO powders with narrow size distribution are greatly required in a subsequent sintering process to obtain uniform grain size without abnormal grain growth in BTO ceramics. Among a number of synthesis routes, the sol–gel method has been recognized as a promising and efficient route to prepare nanocrystalline BTO powders with narrow size distribution. It also offers several potential advantages over conventional solid-state reaction, such as higher purity, better chemical homogeneity, ease of processing, and controllable particle size [18, 19]. Until now, there are a huge number of published works made by researchers to synthesize BTO nanopowders by sol–gel method. However, the preparation and investigation on sol–gel-derived BTO bulk ceramics have been rarely reported in literature. A large majority of publications have only been focused on how to control size and crystal structure of BTO nanopowders without further investigation of sintered-bulk BTO ceramics [19–23]. Moreover, considerable attention has also been directed toward the preparation of sol–gelderived BTO films on various kinds of substrates rather than bulk form [24–27]. It is known that the distinct forms of BTO (i.e., film and bulk) exhibit difference in dielectric and ferroelectric properties. Therefore, dual measurements of dielectric and ferroelectric properties of sol–gel-derived BTO bulk ceramics with different grain sizes are highly needed to promote the progress in this field. In this work, we aim to study the dielectric and ferroelectric properties of fine and coarse-grained BTO ceramics. The BTO nanopowders were firstly synthesized by a sol–gel method using a calcination temperature of 800 °C. The sol–gel-derived BTO powders were subsequently sintered at 1150 and 1350 °C to produce fine and coarsegrained BTO ceramics, respectively. The morphological and structural properties of BTO ceramics were investigated by scanning electron microscopy (SEM) and X-ray diffraction (XRD), respectively. Moreover, comprehensive measurements on dielectric and ferroelectric properties of the BTO ceramics were carried out and compared with other published works. 2 Experimental details purity ≥ 99.9%) were purchased from RCI LabScan. All of the chemicals were analytical grade and used without further purification. 2.2 Synthesis of BTO nanopowders by sol–gel method Barium acetate was firstly dissolved in warm acetic acid under vigorous stirring. Methanol was then added into a clear solution with the acetic acid-to-methanol ratio of 1:2. Subsequently, an equimolar amount of titanium (IV) butoxide was added slowly into the mixture. The solution was kept vigorous stirring at room temperature until it became a transparent gel. The gel was dried in a vacuum oven at 100 °C for 12 h to obtain a dried gel. The dried BTO gel was then calcined at 800 °C for 80 min in an electrical furnace under air atmosphere. The calcination at 800 °C is at least temperature to obtain single-phase BTO powders without impurity and intermediate phases (Fig. S1). The sol–gel-derived BTO powders calcined at 800 °C is hereafter denoted as BTO-800. 2.3 Preparation of fine and coarse‑grained BTO ceramics The calcined BTO powders with a binder were pressed vertically into a pellet shape (10 mm diameter) using a force of 10 tons for 10 min. The sintering process was performed by placing the BTO pellets in an electrical furnace. The temperature increased from room temperature to 300 °C with a heating rate of 2 °C/min and soaked for 2 h. Then, the temperature increased to 550 °C with a heating rate of 2 °C/min and soaked for 5 h to completely remove the binder. After that, the BTO pellets were sintered at 1150 and 1350 °C for 2 h with a heating rate of 4.5 °C/min from 550 °C. After sintering, the specimens were naturally cooled to room temperature. The sintered BTO ceramics were then polished on sand papers (400, 800 and 1200 grit) to obtain a flat and parallel surface, followed by ultrasonically cleaning in acetone and ethanol, respectively. The final thickness of BTO ceramics was about 1 mm. The BTO ceramics sintered at 1150 and 1350 °C are hereafter denoted as BTO-1150 (fine grains) and BTO-1350 (coarse grains), respectively. 2.1 Materials 2.4 Characterizations Barium acetate (Ba(CH3COO) 2, purity ≥ 99.999%) and titanium (IV) butoxide (Ti(O(CH2)3CH3)4, purity ≥ 97.0%) were purchased from Sigma Aldrich. Gracial acetic acid ­( CH 3COOH, purity ≥ 99.9%) and methanol ­(CH 3OH, The morphology of sol–gel-derived BTO powders and sintered BTO ceramics was investigated with a JEOL JSM-6480LV scanning electron microscope (SEM) at an acceleration voltage of 15 kV. The XRD patterns were 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. A comparative study of dielectric and ferroelectric properties of sol–gel-derived B ­ aTiO3… recorded on a Rigaku Dmax 2002 diffractrometer with Cu Kα radiation (λ = 0.15406 nm) operated at 40 kV and 30 mA to identify the crystal and phase structure. The density of sol–gel-derived BTO powders was measured using a Quantachrome Ultrapycnometer 1000 under helium purge at a pressure of 20 psi. 2.5 Dielectric and ferroelectric measurements For the preparation of specimens, gold electrodes were deposited on both-side surfaces of the BTO ceramic pellets by a direct current (DC) sputtering. The dielectric properties of the BTO ceramics were measured using a Hewlett–Packard 4194A impedance/gain phase analyzer. The measurements were carried out in the frequency range of 100 Hz–10 MHz and the temperature range of − 45 to 180 °C. The dielectric constant (εr) was calculated from the measured capacitance using the following equation: 𝜀r = CA , 𝜀0 d (1) where C is the capacitance (F), ε0 is the dielectric constant of free space (8.85 × 10−12 F/m), A is the electrode area (­ m2), and d is the thickness of specimen (m). The polarization–electric field (P–E) loops of the BTO ceramics were recorded at room temperature using a Radiant Technology RT66A standardized ferroelectric measurement test system with an applied voltage from ± 1000 to ± 4000 V. 3 Results and discussion 3.1 Morphology The SEM image of sol–gel-derived BTO powders (BTO800) is displayed in Fig. 1a. The BTO-800 revealed aggregates of uniform round-shaped particles with a narrow Page 3 of 8 713 size distribution. The average particle size of BTO-800 was determined to be 95 ± 15 nm, which is very close to commercially available BTO nanopowders, < 100 nm (Sigma Aldrich). For the sintered BTO ceramics, the SEM images of BTO-1150, and BTO-1350 are illustrated in Fig. 1b, c, respectively. An obvious increase in grain size was clearly seen for BTO-1150 and BTO-1350. The average grain size was estimated to be 1.0 ± 0.3 µm for BTO-1150 and substantially increased to 12.2 ± 4.6 µm for BTO-1350. This result indicates that grain size of BTO increased with increasing sintering temperature. 3.2 Crystal structure To analyze the phase and crystal structure, the XRD patterns of BTO-800, BTO-1150 and BTO-1350 are shown in Fig. 2a. For BTO-800, a set of detectable diffraction peaks can be assigned to the BTO phase (JCDPS no. 31-0174) without other crystalline phases. A splitting phenomenon of the diffraction peak, especially 002/200 peaks, is typically used as the identification of ferroelectric BTO with a tetragonal structure [13, 15, 17]. However, a symmetric single peak without splitting feature was observed for all diffraction peaks of BTO-800. This result suggests that the crystal structure in BTO-800 exhibited a cubic structure, rather than a tetragonal structure. The formation of the cubic phase is generally found in case of nano-sized BTO particles [28, 29]. Using a helium pycnometer, the density of BTO-800 was determined to be 5.39 g/cm3, which is about 90% of the bulk density (6.02 g/cm3), indicating the dense nanoparticular structure. After sintering, the evident splitting of the 002 diffraction peak into 002 and 200 peaks was clearly visible for BTO-1150 and BTO1350, as shown in Fig. 2b. The splitting feature was also observed on the 100/001, 101/110, 102/210, 112/211, and 202/220 diffraction peaks, excepting the 111 diffraction peak (JCDPS no. 05-0626). This result confirms that the tetragonal structure of the BTO ceramics Fig. 1 SEM images of a BTO-800, b BTO-1150 and c BTO-1350 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. 713 Page 4 of 8 G. Panomsuwan, H. Manuspiya Fig. 2 a XRD patterns of BTO800, BTO-1150, and BTO1350. b Enlarged view of XRD patterns for all samples around 111 and 002/200 diffraction peaks was formed well after sintering at high temperature. The peak intensity ratio (I002/I200) of BTO-1150 and BTO-1350 was nearly close to 1:2, which is in agreement with tetragonal BTO reported in several previous reports [28, 30, 31]. In addition, the diffraction peaks of both sintered BTO ceramics were intense and sharp, indicating their good crystallinity. The crystallite size (D) was determined using the Scherrer’s equation based on the position (2θ) and full-width at half maximum (β) of the 111 diffraction peak as follows: D= K𝜆 , 𝛽 cos 𝜃 (2) where λ is the wavelength of X-ray (0.15406 nm) and K is the Scherrer constant (herein 0.94). The 111 diffraction peak was chosen for calculating crystallite size owing to its no splitting feature. The calculated crystallite size of BTO-800, BTO-1150, and BTO-1350 was 27.5, 43.1, and 46.3 nm, respectively. This result indicates that the crystallite size of BTO became larger after sintering; however, only a slight change was observed between BTO1150 and BTO-1350. In association with the SEM results, the average grain sizes of the sintered BTO ceramics were much larger than their crystallite size calculated from the Scherrer’s equation. This means that each individual grain of the sintered BTO ceramics was constituted of several ferroelectric crystalline domains. Furthermore, in-plane (a) and out-of-plane lattice constants (c) of BTO-800, BTO-1150, and BTO-1350 were calculated using the Bragg’s law from the 200 and 002 diffraction peaks, respectively. In case of BTO-800, the calculated a and c values were both equal to 0.4010 nm owing to its inseparable 002 diffraction peak (cubic structure) as mentioned above. For BTO-1150 and BTO-1350, the c values were larger than a values because of their tetragonal structure (c > a). Tetragonality (c/a ratio) of the BTO-1150 and BTO-1350 had the almost same value of about 1.0105. The results of the lattice constant, crystallite size, and tetragonality are summarized in Table 1. Based the SEM and XRD results, it suggests that although sintering temperature had a significant influence in substantially increasing grain size, only a slight change in crystallite size and tetragonality was observed for BTO-1150 and BTO-1350. 3.3 Dielectric properties The dielectric properties of BTO-1150 and BTO-1350 in the frequency range from 100 Hz to 10 MHz at room temperature are shown in Fig. 3a, b, respectively. At a frequency of 1 kHz, the dielectric constant of BTO-1150 was 1502 with tanδ of 0.016, while the dielectric constant and tanδ of BTO-1350 were 1082 and 0.021, respectively. A similar changing trend of dielectric constant and tanδ with varying frequency was noticed for both BTO ceramics. In the range of 100 Hz–1 MHz, the dielectric constant decreased slowly, while tanδ varied slightly. With a further increase of frequency above 1 MHz, an abrupt drop in the dielectric constant and a rapid increase in tanδ were observed. This characteristic behavior is attributed to the orientational dielectric Table 1 Summary of in-plane lattice constant (a), out-of-plane lattice constant (c), tetragonality (c/a), unit-cell volume (V), crystal structure, and crystallite sizes (D) of BTO-800, BTO1150, and BTO-1350 Sample A (nm) c (nm) c/a V ­(nm3) Crystal structure D (nm) BTO-800 BTO-1150 BTO-1350 0.4010 0.3994 0.3995 0.4010 0.4036 0.4037 1.0000 1.0105 1.0105 0.0645 0.0644 0.0644 Cubic Tetragonal Tetragonal 27.5 43.1 46.3 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. A comparative study of dielectric and ferroelectric properties of sol–gel-derived B ­ aTiO3… Page 5 of 8 713 Fig. 3 Dielectric constant and tanδ in the frequency range from 1 kHz to 10 MHz at room temperature of a BTO-1150 and b BTO-1350 Fig. 4 Dielectric constant of BTO-1150 and BTO-1350 as a function of temperature from − 45 to 180 °C in the frequency range from 100 Hz to 1 MHz relaxation owing to the domain-wall vibration induced by external electric field [12]. More detailed information on dielectric properties of the BTO ceramics with fine and coarse grains was further drawn by analyzing the temperature-dependent dielectric constant during cooling, as shown in Fig. 4. There were two peaks in each dielectric constant curve within the measured temperature range from − 45 to 180 °C, corresponding to the tetragonal-to-cubic (T–C) phase transition and the orthorhombic-to-tetragonal (O–T) phase transition of the BTO. It was noticed that the dielectric constant of BTO-1150 and BTO-1350 reached the maximum values of 2500 and 8730, respectively (1 kHz), at the T–C phase transition or Curie temperature (TC = 125 °C). With a further decrease in temperature, a smaller and broader peak corresponding to the O–T transition appeared at 0 °C for BTO-1350, while a shift toward higher temperature was found for BTO-1150. From temperature-dependent dielectric constant, it was evident that the dielectric peaks at both phase transitions of BTO-1150 became more diffused and broader than those of BTO-1350. The diffused feature at phase transition of BTO-1150 can be explained by distribution of distributed TC as a result of inhomogeneity due to the different types of defects and various local densities existing in BTO-1150. In addition, another important aspect is that dielectric constant of BTO-1150 was greater than that of BTO-1350 at temperature below TC. Such a dielectric behavior is almost similar to the results reported by Arlt et al. [11] and Lee et al. [32]. Higher dielectric constant of BTO-1150 arises from its higher internal stress in an individual fine grain as compared to BTO-1350. Our result is in agreement with those obtained by other works (Table 2) that the dielectric constants at room temperature were in the range of about 700–1500 for a broad grain size between 0.8 and 40 μm [33–41]. It can conclude that although sol–gel-derived BTO ceramics have different grain sizes, a difference in precursors and preparation conditions do not affect significantly to their dielectric properties at room temperature. The additional chemicals may be needed in the sol–gel synthesis for further enhancement of dielectric constant. For example, Cui’s group employed the various kinds of organic acids as surfactants (i.e., decanoic acid, decanedioic acid, cetylic acid, and stearic acid) in the sol–gel synthesis of BTO powders [40, 41]. They found that the dielectric constant at room temperature enhanced up to 2000–4000 depending on type of organic acids since the relative density of the ceramic was quite close to theoretical density. In contrast, unlike at room temperature, the dielectric constant at TC of BTO ceramics varied broadly from 2500 to 9000, which strongly depended on grain size (see Table 2). Therefore, the diffused behavior at the T–C phase transition of sol–gel-derived BTO ceramics can be manipulated by adjusting grain size. Moreover, there 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. Ba(CH3COO)2/TTIP Ba(CH3COO)2/TTIP Ba(CH3COO)2/TTIP BaCO3/TBOT Ba(CH3COO)2/TTIP Ba(NO3)2 ·8H2O/Ti/H2O2 Ba(CH3COO)2/TTIP Ba(CH3COO)2/TBOT Ba(CH3COO)2/TBOT Ba(CH3COO)2/TBOT Sharma et al. [33] Sharma et al. [34] Lobo et al. [35] Deshpande et al. [36] Li et al. [37] Devi et al. [38] Mahmood et al. [39] Yu et al. [40] Cui et al. [41] This work 800 °C/1.3 h 900 °C/2 h 900 °C 850 °C/4 h 900 °C/2 h 750 °C/2 h 700 °C/1 h 500 °C/6 h 750 °C/6 h 700 °C/2 h Calcination condition 1350 °C/2 h 1250 °C/2 h 1300 °C/2 h 1300 °C/2 h 1350 °C/2 h 1280 °C/4 h 1200 °C/2 h 1300 °C/2 h 1400 °C/2 h 1250–1350 °C/4 h 1300 °C/6 h 1250 °C/2 h Sintering condition TTIP titanium (IV) tetraisopropoxide ­(C12H28O4Ti), TBOT titanium (IV) butoxide ­(C16H36O4Ti) Precursor References – – 0.8 5–15 0.8 20 40 – 18 0.5 2.0 0.7 1.5 2.0 45 1.0 12.2 Grain size (μm) 700 1170 – 1280 1082 1040 1021 1452 800 2169 3270 3120 3392 3278 4569 1502 1082 εr at 25 °C Table 2 Precursors, calcination and sintering conditions of sol–gel-derived BTO bulk ceramics and their dielectric properties – 0.011 – 0.016 0.028 0.021 0.024 0.043 – 0.030 0.018 0.029 0.032 0.033 0.027 0.016 0.021 tanδ at 25 °C 2500 4370 6200 7200 1642 6170 5190 4092 5700 7955 8960 6650 7470 8355 8970 2500 8730 εmax at TC 1 kHz 1 kHz 1 kHz 1 MHz 1 kHz 1 kHz 1 kHz 10 kHz 1 kHz 100 kHz Frequency 713 Page 6 of 8 G. Panomsuwan, H. Manuspiya 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. A comparative study of dielectric and ferroelectric properties of sol–gel-derived B ­ aTiO3… Page 7 of 8 713 Fig. 5 P–E loops measured at room temperature of a BTO-1150 and b BTO-1350 was no significant change in dielectric constant for both BTO ceramics as the frequency increased from 100 Hz to 1 MHz along temperature range investigated, indicating their frequency independent behavior or non-relaxor ferroelectric response. and BTO-1350 is mainly attributed to the difference in the grain size and dense in microstructure. It can conclude that BTO-1350 is more suitable for ferroelectric device applications than BTO-1150 owing to its ferroelectric domain switching behavior with low loss. 3.4 Ferroelectric properties To further evaluate the ferroelectric behavior of sintered BTO ceramics, the measurements of P–E loops were carried out at room temperature, as shown in Fig. 5. The P–E loop of BTO-1150 revealed an ellipse shape with an unsaturated polarization, which is the characteristic feature of a lossy hysteresis loop [42, 43]. It implies that BTO-1150 possessed high leakage current or low resistance possibly due to its low dense microstructure and the presence of porosities. Unlike in fine-grained BTO-1150, the P–E loop of BTO-1350 showed a hysteresis loop with well-saturated polarization at the applied voltage greater than ± 3000 V. This feature is a clear evidence of ferroelectric domain switching in BTO1350. With an applied voltage of ± 4000 V, the remnant polarization (Pr) of BTO-1350 reached 20.6 μC/cm2, while the coercive fields (Ec) was 4.8 kV/cm. The Ec of BTO-1150 was found at 25.5 kV/cm, which is much greater than that of BTO-1350. It is known that Ec refers to an external electric field required for polarization reversal in ferroelectric materials. Therefore, it can suggest that a larger energy or higher external electric field is required to reorient the domain walls in BTO-1150, as compared to BTO-1350. High density of grain boundaries and defects of fine-grained BTO-1150 can cause the pinning effects on the movement of domain walls, thus inducing high Ec values. For the BTO-1350 having coarse grains, low density of grain boundary has a dilution effect, resulting in a weak influence on its ferroelectric properties. The difference in the P–E loops between BTO-1150 4 Conclusions The BTO nanopowders were successfully synthesized via sol–gel method followed by subsequent calcination at 800 °C. The synthesized BTO powders exhibited a cubic structure with the particle size of less than 100 nm. The BTO-1150 and BTO-1350 had an average grain size of about 1 and 12 µm, respectively. They showed a tetragonal structure with high tetragonality (c/a) of 1.0105. The frequency dependence on dielectric properties of BTO-1150 and BTO-1350 revealed the relaxation behavior at high frequency above 1 MHz. The dielectric constant of BTO-1150 was less sensitive to change with temperature than BTO1350. The T–C phase transition or TC occurred at 125 °C for both BTO ceramics. Room-temperature dielectric constant of fine-grain BTO was higher than that of coarse-grained BTO due to the grain size effect. A well-defined P–E hysteresis loop was observed only for BTO-1350 with coarse grain size. In contrast, a lost hysteresis loop was observed for BTO-1150 owing to its large leakage current. Our results in this work can serve as a useful information and reference on the dielectric and ferroelectric properties of sol–gel-derived BTO bulk ceramics as well as a helpful guideline to the researchers in this field. Acknowledgements This work was financially supported by Kasetsart University Research and Development Institute (KURDI) and Research Grant for New Scholar from Thailand Research Fund (MRG-6180095). 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. 713 Page 8 of 8 G. Panomsuwan, H. Manuspiya References 23. Y.T. Wu, X.F. Wang, C.L. Yu, E.Y. Li, Mater. Manuf. Process. 27, 1329 (2011) 24. H. Kumazawa, K. Masuda, Thin Solid Films 353, 144 (1999) 25. M. Manso-Silván, L. Fuentes-Cobas, R.J. Martín-Palma, M. Hernández-Vélez, J.M. Martínez-Duart, Surf. Coat. Technol. 151–152, 118 (2002) 26. S. Song, J. Zhai, X. Yao, Mater. Sci. Eng. B 145, 28 (2007) 27. R. Ashiri, A. Nemati, M.S. Ghamsari, Ceram. Int. 40, 8613 (2014) 28. X. Li, W.-H. Shih, J. Am. Ceram. Soc. 80, 2844 (1997) 29. T. Yamamoto, H. Niori, H. Moriwake, Jpn. J. Appl. Phys. 39, 5683 (2000) 30. K.-C. huang, T.-C. Huang, W.-F. Hsieh, Inorg. Chem. 48, 9180 (2009) 31. H. Maiwa, J. Ceram. Soc. Jpn. 121, 655 (2013) 32. B.W. Lee, K.H. Auh, J. Mater. Res. 10, 1418 (1995) 33. H.B. Sharma, R.P. Tandon, A. Mansingh, R. Rup, J. Mater. Sci. Lett. 12, 1795 (1993) 34. H.B. Sharma, H.N.K. Sarma, A. Mansingh, J. Mater. Sci. 34, 1385 (1999) 35. R.P.S.M. Lobo, N.D.S. Mohallem, R.L. Moreira, J. Am. Ceram. Soc. 78, 1343 (1995) 36. S.B. Deshpande, P.D. Godbole, Y.B. Khollam, H.S. Potdar, J. Electroceram. 15, 103 (2005) 37. W. Li, Z. Xu, R. Chu, P. Fu, J. Hao, J. Alloys Compd. 482, 137 (2009) 38. Ch.S. Devi, M. Vithal, G.S. Kumar, G. Prasad, J. Mater. Sci. Mater. Electron. 22, 1855 (2011) 39. A. Mahmood, A. Naeem, Y. Iqbal, T. Mahmood, A. Ullah, J. Mater. Sci. Mater. Electron. 26, 5635 (2015) 40. P. Yu, X. Wang, B. Cui, Scr. Mater. 57, 623 (2007) 41. B. Cui, P. Yu, X. Wang, J. Alloys Compd. 459, 589 (2008) 42. J.-H. Park, B.-K. Kim, J.G. Park, I.-T. Kim, H.-J. Je, Y. Kim, S.J. Park, Ferroelctrics 230, 151 (1999) 43. B. Dhanalakshmi, K. Pratap, B. ParvatheeswaraRao, P.S.V. Subba Rao, J. Alloys Compd. 675, 193 (2016) 1. G.H. Haertling, J. Am. Ceram. Soc. 82, 797 (1999) 2. M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G.A. Rossetti Jr., J. Rödel, Appl. Phys. Rev. 4, 041305 (2017) 3. Q. Wang, F. Li, J. Phys. D Appl. Phys. 51, 255301 (2018) 4. J. Yuk, T. Troczynski, Sens. Actuator B Chem. 94, 290 (2003) 5. A. Koka, H.A. Sodano, Nat. Commun. 4, 2682 (2013) 6. G. Panomsuwan, O. Takai, N. Saito, Appl. Phys. A 108, 337 (2012) 7. Y. Tan, J. Zhang, Y. Wu, C. Wang, V. Koval, B. Shi, H. Ye, R. McKinnon, G. Viola, H. Yan, Sci. Rep. 5, 9953 (2015) 8. H. Ghayour, M. Abdellahi, Powder Technol. 292, 84 (2016) 9. W.R. Buessem, L.E. Cross, A.K. Goswami, J. Am. Ceram. Soc. 49, 33 (1966) 10. K. Kinoshita, A. Yamaji, J. Appl. Phys. 47, 371 (1976) 11. G. Arlt, D. Henning, G. De With, J. Appl. Phys. 58, 1619 (1985) 12. T. Hoshina, K. Takizawa, J. Li, T. Kasama, H. Kakemoto, T. Tsurumi, Jpn. J. Appl. Phys. 47, 7607 (2008) 13. L. Curecheriu, M.T. Buscaglia, V. Buscaglia, Z. Zhao, L. Mitoseriu, Appl. Phys. Lett. 97, 242909 (2010) 14. Y. Huan, X. Wang, J. Fang, L. Li, J. Eur. Ceram. Soc. 34, 1445 (2014) 15. U. Hasenkox, S. Hoffmann, R. Waser, J. Sol Gel Sci. Technol. 12, 67 (1998) 16. P. Pinceloup, C. Courtois, A. Leriche, B. Thierry, J. Am. Ceram. Soc. 82, 3049 (1999) 17. M.C. Cheung, H.L.W. Chan, C.L. Choy, J. Mater. Sci. 36, 381 (2001) 18. P. Yu, B. Cui, Q. Shi, Mater. Sci. Eng. A 473, 34 (2008) 19. A. Kareiva, S. Tautkus, R. Rapalaviciute, J.-E. Jørgensen, B. Lundtoft, J. Mater. Sci. 34, 4853 (1999) 20. C. Lemoine, B. Gilbert, B. Michaux, J.-P. Pirard, A.J. Lecloux, J. Non-Cryst. Solid 175, 1 (1994) 21. R. Kavian, A. Saidi, J. Alloy. Compd. 468, 528 (2009) 22. Y. Hao, X. Wang, H. Zhang, L. Guo, L. Li, J. Sol Gel Sci. Technol. 67, 182 (2013) 13 Content courtesy of Springer Nature, terms of use apply. Rights reserved. Terms and Conditions Springer Nature journal content, brought to you courtesy of Springer Nature Customer Service Center GmbH (“Springer Nature”). Springer Nature supports a reasonable amount of sharing of research papers by authors, subscribers and authorised users (“Users”), for smallscale personal, non-commercial use provided that all copyright, trade and service marks and other proprietary notices are maintained. By accessing, sharing, receiving or otherwise using the Springer Nature journal content you agree to these terms of use (“Terms”). For these purposes, Springer Nature considers academic use (by researchers and students) to be non-commercial. These Terms are supplementary and will apply in addition to any applicable website terms and conditions, a relevant site licence or a personal subscription. These Terms will prevail over any conflict or ambiguity with regards to the relevant terms, a site licence or a personal subscription (to the extent of the conflict or ambiguity only). For Creative Commons-licensed articles, the terms of the Creative Commons license used will apply. We collect and use personal data to provide access to the Springer Nature journal content. We may also use these personal data internally within ResearchGate and Springer Nature and as agreed share it, in an anonymised way, for purposes of tracking, analysis and reporting. We will not otherwise disclose your personal data outside the ResearchGate or the Springer Nature group of companies unless we have your permission as detailed in the Privacy Policy. While Users may use the Springer Nature journal content for small scale, personal non-commercial use, it is important to note that Users may not: 1. use such content for the purpose of providing other users with access on a regular or large scale basis or as a means to circumvent access control; 2. use such content where to do so would be considered a criminal or statutory offence in any jurisdiction, or gives rise to civil liability, or is otherwise unlawful; 3. falsely or misleadingly imply or suggest endorsement, approval , sponsorship, or association unless explicitly agreed to by Springer Nature in writing; 4. use bots or other automated methods to access the content or redirect messages 5. override any security feature or exclusionary protocol; or 6. share the content in order to create substitute for Springer Nature products or services or a systematic database of Springer Nature journal content. In line with the restriction against commercial use, Springer Nature does not permit the creation of a product or service that creates revenue, royalties, rent or income from our content or its inclusion as part of a paid for service or for other commercial gain. Springer Nature journal content cannot be used for inter-library loans and librarians may not upload Springer Nature journal content on a large scale into their, or any other, institutional repository. These terms of use are reviewed regularly and may be amended at any time. Springer Nature is not obligated to publish any information or content on this website and may remove it or features or functionality at our sole discretion, at any time with or without notice. Springer Nature may revoke this licence to you at any time and remove access to any copies of the Springer Nature journal content which have been saved. To the fullest extent permitted by law, Springer Nature makes no warranties, representations or guarantees to Users, either express or implied with respect to the Springer nature journal content and all parties disclaim and waive any implied warranties or warranties imposed by law, including merchantability or fitness for any particular purpose. Please note that these rights do not automatically extend to content, data or other material published by Springer Nature that may be licensed from third parties. If you would like to use or distribute our Springer Nature journal content to a wider audience or on a regular basis or in any other manner not expressly permitted by these Terms, please contact Springer Nature at onlineservice@springernature.com