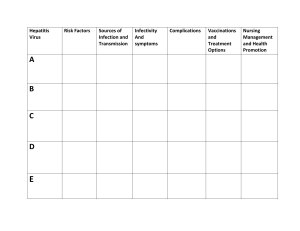
СМОЛЕНСКИЙ ГОСУДАРСТВЕННЫЙ МЕДИЦИНСКИЙ УНИВЕРСИТЕТ Кафедра патологической анатомии Ю.С. Корнева, Л.И. Скробут PATHOLOGY OF INFECTIOUS DISEASES Учебное пособие Издание одобрено и рекомендовано к печати Центральным методическим советом Смоленского государственного медицинского университета Смоленск, 2015 ББК 55.14 УДК 616.9 (075.8) Рецензенты: Козлов Дмитрий Васильевич - доктор медицинских наук, профессор кафедры патологической анатомии ГБОУ ВПО Смоленский государственный медицинский университет Минздрава России, заведующий отделение клинической патологии № 2 ОГБУЗ «Смоленского областного института патологии»; Молчанов Владимир Васильевич - кандидат медицинских наук, доцент кафедры патологической анатомии ГБОУ ВПО Смоленский государственный медицинский университет, заведующий отделением клинической патологии №3 ОГБУЗ «Смоленского областного института патологии» Николаева Татьяна Владимировна - заведующая кафедрой иностранных языков ГБОУ ВПО Смоленский государственный медицинский университет Минздрава России Корректор: Ковалькова Марина Валерьевна - старший преподаватель кафедры иностранных языков ГБОУ ВПО Смоленский государственный медицинский универсиетет Минздрава России Корнева Ю.С., Скробут Л.И. Pathology of infectious diseases: учебное пособие / Ю.С. Корнева, Л.И. Скробут: Под научной редакцией проф. А.Е. Доросевича. – Смоленск: СГМА, 2015. – 84 с. Учебное пособие посвящено вопросам инфекционной патологии. В нем отражены вопросы этиологии, пути передачи, классификации, морфология и осложнений различных наиболее актуальных инфекционных заболеваний, таких как: острые респираторные вирусные инфекции, детские инфекции, кишечные инфекции, особо опасные инфекции, ВИЧ, сифилис, туберкулез. Отдельный раздел посвящен современным клиникомофрологическим аспектам патогенеза сепсиса. Пособие предназначено для студентов третьего курса факультета иностранных учащихся медицинского вуза для подготовки к практическим занятиям и самостоятельной работы. Учебное пособие рекомендовано Центральным методическим советом ГОУ ВПО СГМА Минздрава России № 2 «1» декабря 2015 г. ББК 55.14 УДК 616.9 (075.8) © Корнева Ю.С., Скробут Л.И. 2015 © ГБОУ ВПО СГМУ Минздрава РФ, 2015 2 SMOLENSK STATE MEDICAL UNIVERSITY Department of Pathological Anatomy Yu. S. Korneva, L.I. Skrobut PATHOLOGY OF INFECTIOUS DISEASES Approved and recommended for publishing by the Didactic Board of the Smolensk State Medical University Smolensk 2015 3 CONTENTS INTRODUCTION…………………………………………………………… 5 CHILDHOOD INFECTIONS:……………….............................................. 6 Measles…………………………………………………………….………… 6 Chickenpox…………………………………………………………………. 7 Shingles……………………………………………………………………….. 8 Mumps…..……………………………………................................................. 9 Rubella………… ……………………………….............................................. 10 Infectious Mononucleosis…………………………………………………… 11 Scarlet Fever……………………………………………………...…………... 12 Pertussis………………………………………………………………….…… 12 Diphtheria……………………………………………………………….……. 13 Meningococcal Infection…………………………………….………..……… 16 TUBERCULOSIS………………………………………………..…..……… 21 SYPHILIS……………………………………………………………..…….. 31 INTESTINAL INFECTIONS…………………………………………….. 35 Typhoid Fever………………………………………………………………. 35 Cholera…………………………………………………………………….... 38 Dysentery…………………………………………………………………… 41 SEPSIS……………………………………………………………………... 43 ACUTE RESPIRATORY INFECTIONS………………………………….. 57 Influenza …………………………………………………………………… 57 Parainfluenza …………………………………………………………………. 59 Adenoviral Infection………………………………………………………. 60 HIGHLY CONTAGEOUS INFECTIONS ……………………………… 61 Plague ………………………………………………………………………. 61 Anthrax …………………………………………………………………….. 64 AIDS ………………………………………………………………………... 69 REFERENCES…………….……………………………………................. 80 4 Введение Инфекционный болезни на протяжении многих тысячелетий являлись основной причиной смерти в популяции. В настоящее время, несмотря на широкое внедрение различных вариантов антимикробной химиотерапии и вакцинации, заболеваемость и смертность, связанная с различными микроорганизмами, остается высокой. Поэтому врачу любой специальности необходимы знания об этиологии и патогенезе, а также эпидемиологии и возможных осложнениях различных инфекционных заболеваний. Цель данного учебного пособия – дать студентам необходимые теоретические основы этиопатогенеза, морфогенеза, а также элементы эпидемиологии различных инфекционных заболеваний. После изучения инфекционной патологии: Студент должен знать: понятия этиологии, патогенеза, морфогенеза, патоморфоза болезни, нозологии, принципы классификации инфекционных болезней; характерные изменения внутренних органов при наиболее значимых инфекционных заболеваниях; Студент должен уметь: обосновать характер патологического процесса и его клинических проявлений; анализировать, применительно к инфекционным заболевания, аспекты общей патологии и современные теоретические концепции и направления в медицине; описать морфологические изменения изучаемых макроскопических, микроскопических препаратов; осуществлять сопоставление морфологических и клинических проявлений болезней на всех этапах их развития; диагностировать причины, патогенез и морфогенез болезней, их проявления, осложнения и исходы, а также патоморфоз, а в случае смерти — причину смерти и механизм умирания (танатогенез); Студент должен приобрести навыки: макроскопической диагностикой инфекционного процессы; микроскопической (гистологической) диагностикой инфекционных процессов; навыками сопоставления морфологических и клинических проявлений болезней; методами клинико-анатомического анализа вскрытия, исследования биопсийного и операционного материала. 5 MEASLES (Koрь) It is a highly contagious disease, characterized by fever, intoxication, catarrhal inflammation, enanthema (rash on mucous membranes) and exanthema (rash on the skin). ETIOLOGY and TRANSMISSION The disease is caused by a single-stranded RNA virus of the Paramyxovirus family (Polinosa morbillarum). Nowadays it is rare in developed countries due to widespread immunization, but epidemics of the disease usually occur in developing countries where it can be fatal to malnourished children. Measles provides lifelong immunity. Humans are the natural host and reservoir of the virus. The disease is transmitted by respiratory droplets during breathing, coughing or sneezing. PATHOGENESIS: The virus affects epithelium of the respiratory tract, GIT and causes anergia. Primary replication of the virus occurs in the respiratory epithelial cells, later the virus spreads to the local lymph nodes, and then to the blood, resulting in viremia. STAGES OF THE DISEAES: INCUBATION PERIOD: 10-21 days , 14 days on an average PRODROME: characterized by high fever, malaise, anorexia and ―Three C‘s‖: Cough, Coryza (rhinorrhea), and Conjunctivitis. HEIGHT OF THE DISEASE: Two days after the start of the illness Koplik spots appear. These are numerous small white spots on a granular red base on the mucous membrane of the cheeks, opposite the back teeth (enanthema). Histologically areas of micronecrosis with desquamation are revealed. After 2 days the typical pink or red maculopapular rash (exanthema) appears, starting on the face and neck and slowly spreading down to the hands and feet. The rash therefore appears 4 days after the child first feels unwell. After a few days the rash fades, symptoms gradually resolve, and the child feels better. Mild desquamation and brown staining of the skin follow the rash on the 7- 14th days. Histologically inflammation of the skin with the presence of giant cells (WarthinFinkeldey cells, which contain up to 100 nuclei, eosinophilic nuclear and cytoplasmic inclusion bodies) and exudation in the epidermis are revealed. In the respiratory tract catarrhal inflammation of the mucous membranes of the pharynx and trachea, foci of necrosis in the bronchi with the presence of 6 multinucleated cells in the epithelium are present. Squamous metaplasia in the bronchi is common. If the lungs are affected by the virus, pneumonia is interstitial and giant cells are present in the inflammatory infiltrate. Encephalitis may develops in 1/1000 cases. COMPLICATIONS: 1. False croup – stenosis of the larynx due to the inflammation and swelling with asphyxia. 2. Secondary bacterial infections of the respiratory tract, skin, GIT, urinary tract, ear are possible due to anergia. Due to the depressing of the immune system, other infections such as herpes stomatitis and adenoviral pneumonia are also possible. HIV infection may rapidly progress to AIDS while tuberculosis is often reactivated following measles. The Mantoux skin test may be negative despite active tuberculosis develops a few months after measles. 3. Keratitis (inflammation of the cornea) with possible corneal ulcers due to measles or secondary herpes virus. DISEASES CAUSED BY VARICELLA-ZOSTER VIRUS: CHICKENPOX (Ветрянка (also called Varicella) and SHINGLES (Опоясывающий лишай (also called Herpes Zoster) ETIOLOGY and TRANSMITTION Chickenpox is caused by the Varicella-zoster virus – a member of herpes virus family, a double-stranded DNA virus. Like measles, it is highly infectious and is transmitted from person to person by droplet spread. Chickenpox is most infectious at the time when rash appears. Following chickenpox, the same virus may remain silent (dormant) in the body for many years before being reactivated to result in Shingles. Children can therefore also be infected and get chickenpox from an adult with shingles. Then disease is common during late winter and early spring. In addition to airborne, transmission can be contact or vertical. In case of transplacental transmission the newborn can develop Generalized Varicella if the mother contacted a sick person 5 days before delivery or develops Congenital Varicella syndrome if the mother became infected on 6-20 weeks of gestation, which includes hypoplasia of the limbs, blindness , skin lesions, and brain damage. CHICKENPOX PATHOGENESIS Primary infection develops in the nasopharynx through droplet inoculation. Local replication starts in the nasopharynx, causing viremia and dissemination by circulating mononuclear cells. 7 Incubation period: 10-21 days, 12-14 days on an average. The child becomes pyrexial and feels generally unwell. Within hours rash appears on the face, scalp, chest, back and abdomen. The rash starts as a pink macula (spot) which soon becomes a papule (palpable) and then a vesicle (with clear fluid) which progresses to a pustule (containing pus) and finally results in a scab. Lesions appear in multiple waves. Different elements exist simultaneously. The progression takes 1–2 days and the rash, which is very itchy, appears in crops in about 5 days. The vesicles also appear on mucous membranes in the mouth with consequent ulceration. Unless there is secondary infection, the rash does not leave scars. Specific atypical and severe forms of Chickenpox are: Bullous – formation of large blisters Pustular –secondary purulent inflammation of the elements Hemorrhagic – blisters filled with blood Gangrenous – formation of necrosis on the skin and then dirty ulcers Generalized form - with the involvement of internal organs. Such severe forms usually affect children with immunosuppression, especially in case of leukemia or treated with corticosteroids or cytostatics. COMPLICATIONS Usually the illness is mild and is not complicated. Pneumonia and encephalitis or secondary bacterial infections of the skin and soft tissue (phlegmone) are possible. HERPES ZOSTER (SHINGLES) It is a disease characterized by painful skin rash with blisters in a limited area on one side of the body (left or right), often in a stripe due to dermatomal distribution of the virus. The disease always occurs after chickenpox. Once an episode of chickenpox has resolved, the virus is not eliminated from the body and can go on to cause herpes zoster often many years after the initial infection. ETIOLOGY: Varicella-zoster virus. PATHOGENESIS: After primary infection, Varicella-zoster virus establishes latent infection in perineuronal satellite cells of the dorsal nerve root ganglia. Transcription of viral genes continues during latency, and viral DNA can be demonstrated years after the initial infection. Shingles develops when full virus replication occurs in ganglion cells and the agent travels down the sensory nerve from a single dermatome. The virus is reactivated after being dormant in a sensory dorsal root ganglion. Predisposing factors are decrease of host immunity, radiation/chemotherapy, physical trauma or emotional stress, poor nutrition. INCUBATION PERIOD: 1-3 weeks The hallmark of the disease is SKIN LESIONS: elevated groups of vesicles on an erythematous base, later they form scabs resembling Chickenpox. Linear 8 distribution of eruption along one or two dermatomes, supplied by a spinal or cranial nerve is usual. Most commonly involved dermatomes are supplied by thoracic or lumbar spinal nerves T-3/L-2 and ophthalmic branch of the Trigeminal nerve (forehead). Eruptions are preceded by severe pain, itching, redness, tingling, and hyperesthesia. In severe cases, rash can leave permanent scars, long standing pain (post-herpetic neuralgia, numbness, and skin discoloration). Blisters disappear in 2 weeks, but the pain can continue. PATHOLOGY: The skin lesions in chickenpox and shingles are identical to each other and also to the lesions of herpes simplex virus. Vesicles fill with neutrophils and soon erode to become shallow ulcers. In infected cells, Varicella-zoster virus produces the characteristic cytopathic effect, with nuclear homogenization and intranuclear inclusions. Inclusions are large and eosinophilic and are separated from the nuclear membrane by a clear zone (halo). Multinucleated cells are common. MUMPS (Инфекционный паротит или Свинка) It is a systemic disease characterized by inflammation of the salivary glands and other glands, especially the parotid gland. ETIOLOGY and TRANSMITTION The disease is caused by the Paramyxovirus, RNA virus. The disease is declined due to vaccination. The disease gives lifelong immunity. Humans are the only natural hosts for this virus. The way of transmission is airborne. PATHOGENESIS The virus enters through the mouth and nostrils, replicates in the URT and lymph nodes, then due to viremia spreads to the salivary glands. The virus has tropism to different gland. Also gonads (Epididymoorchitis), the pancreas, meninges may be affected. Fever as well as swelling and tenderness of one or both parotid glands are the main symptoms of mumps. The enlarged parotid glands lift the lower part of the ear. Chewing may be painful due to the swollen glands. Sometimes the submandibular glands (below the jaw) may be involved. PATHOLOGY: Mumps virus causes necrosis of infected cells, associated with a predominantly lymphocytic inflammatory infiltrate. Affected salivary glands are swollen, their ducts lined by necrotic epithelium and their interstitium is infiltrated with lymphocytes. In case of epididymoorchitis the testis can be swollen to three times normal size. The swelling of testicular parenchyma, confined within the tunica albuginea, produces focal infarctions. Mumps orchitis is usually unilateral. COMPLICATIONS 9 Mumps usually has no complications and recovery takes about 1 to 2 weeks. However, mumps may cause very painful orchitis (inflammation of the testes) in postpubertal males, in boys it may result in sterility. Inflammation with the damage of the pancreas may cause diabetes mellitus type 1. Viral meningitis is another rare complication. Rubella / German measles (Краснуха) ETIOLOGY and TRANSMITTION The disease is caused by Togavirus, a single stranded RNA virus. This disease was once a common childhood illness, but its occurrence has been cardinally reduced since vaccine against rubella became available in 1969. Only humans are hosts. Rubella gives lifelong immunity. The way of transmission is airborne. Rubella infects respiratory epithelium, then disseminates through the bloodstream and lymphatics. A person infected with the rubella virus is contagious for about seven days before any symptoms appear and continues to spread the disease for about four days after the appearance of symptoms. Transplacental transmission is possible during first 16 weeks of pregnancy during viremia. Incubation period: 14-21 days PRODOMAL SYMPTOMS: uncommon in children, mainly seen in adolescents: low fever, malaise 1- 4 days, pharyngitis, presence of Forschheimer’s spots – petechiae papules on the soft palate before rash appears. Stages of EXANTHEMA: The first visible sign of rubella is a fine red rash that appears on the face and rapidly moves downward to cover the whole body within 24 hours. The rash lasts for about three days, that is why rubella is sometimes called the three-day measles. Moderate fever and swollen glands, especially in the head (around the ears) and neck, often accompany the rash. Joint pain and sometimes-joint swelling can occur, more often in women. It is quite common to get rubella and not show any symptoms (subclinical infection). Symptoms disappear within three to four days. COMPLICATIONS Most people recover fully with no complications. It can have severe complications for women in their first trimester of pregnancy. Babies may be miscarried or stillborn and a high percentage are born with birth defects. Birth defects are reported to occur in 50% of women who contract the disease during the first month of pregnancy, 20% of those who contract it in the second month, and 10% of those who contract it in the third month. The most common birth defects resulting from congenital rubella infection are eye defects, such as cataracts, glaucoma, and blindness, deafness, congenital heart defects, and mental retardation. Taken together, these conditions are called congenital rubella syndrome (CRS). The risk of birth defects drops after the first trimester, and by the fifth month, there are rarely any complications. 10 INFECTIOUS MONONUCLEOSIS (Инфекционный мононуклеоз) ETIOLOGY and TRANSMISSION: Epstein–Barr Virus (EBV) is a DNA virus, belongs to the group of Herpes virus, has tropism to B-lymphocytes and may persist in them. In addition, the virus is the etiological agent for Berkitts Lymphoma and oropharyngeal carcinoma. EBV spreads via respiratory droplets, during coughing and kissing; the contact way of spreading is possible. Nowadays infectious mononucleosis is considered an etiologically heterogeneous syndrome characterized by the presence of fever, sore throat, lymphadenopathy, and a predominance of atypical lymphocytes on the peripheral blood smear. The majority of cases of IM are caused by EBV or Cytomegalovirus (CMV). It is a self-limited disease. EPIDEMIOLOGY: The disease occurs in all age groups, predominantly in adolescents and young adults. Mild cases can be observed in children. PATHOGENESIS: EBV replicates in the oropharyngeal epithelium. Due to depletion in the epithelium the cells rupture releasing the virus. Selective infection of B-lymphocytes occurs through CD21- receptor, resulting in proliferation of cells in the tonsils, lymph nodes and the spleen. Uncontrolled proliferation of B-cells may be the reason for lymph-proliferative disease (lymphoma). In addition, affection of B-cells disturbs antibody formation, causing secondary immunodeficiency and promoting secondary infections. INCUBATION PERIOD: 30-50 days. MORPHOLOGY: Affection of the throat: pharyngitis (50%) with palatal petecchia, tonsillitis, enlargement of tonsils, covered with whitish exudate. Cervical Lymphadenopathy: enlarged, firm, usually painless lymph nodes Hepatomegaly and Splenomegaly Skin rash seen in 10-15%: polymorphic elements on the chest, abdomen, extremities; the rash increases if penicillin derivatives are used CNS features: Encephalitis, aseptic meningitis, Guillain-Barre syndrome Atypical mononuclears are revealed in blood analysis. COMPLICATIONS are rare. The main serious concern with mono is that the spleen will enlarge and even rupture. Also may be asphyxia due to severe enlargement of tonsils. Impairment of CNS and the pancreas are possible. 11 SCARLET FEVER (Scarlatina) (Скарлатина) ETIOLOGY and TRANSMITTION: Beta-hemolytic streptococcus Group A (Streptococcus pyogenes) that produces erythrotoxigenic toxins. The disease develops in children between 2-10 years old. Most cases are seen in winter and early spring. People with any form of streptococcal infection and carriers are infective. The ways of transmission are airborne or contact. PATHOGENESIS: The portal of the infection is the pharynx, wound or genitalia in puerpera. Streptococcus spreads via lymphogenic and heamatogenic or by intracanalicular way to adjacent structures. Inflammation at first is catarrhal tending to turn purulent. Exotoxin causes intoxication. Incubation period: 1-7 days, 2- 4 days on an average. Prodromal symptoms: fever, sore throat, malaise, myalgia. HEIGHT OF THE DISEASE: Mouth: The pharynx and tonsils are bright red (―scarlet‖ means ―red‖), covered with exudates. Petechia are seen on the tonsils and palate (Forchheimer spots). The tongue is covered with a white coat, after removing it we can see edematous red papilla - ―strawberry tongue‖. Regional lymph nodes are enlarged and painful. Face: Malar flush with the Circumoral pallor is present (Filatov‘s syndrome) Pastia line‘s: linear petechia in the cubital area and the groin Rash is fine, red, and rough-textured; it blanches upon pressure, appears 12–48 hours after the fever, generally starts on the chest, axilla (armpits), and behind the ears, worsening in the skin folds. There are 3 main groups of COMPLICATIONS: 1. SEPTIC: purulent-necrotic changes in the tonsils (tonsillitis), purulent lymphadenitis, otitis, paratonsilar abscess, phlegmon of the neck, sinusitis, bronchitis, pneumonia, and sepsis 2. TOXIC: toxic shock in the toxic form of SF due to releasing exotoxin which blocks sympatic nervous system and corticosteroids. 3. ALLERGIC: due to sensitization on the 2-3rd week after the onset: glomerulonephritis, myocarditis, rheumatic fever, synovitis may develop PERTUSSIS / WHOOPING COUGH (Коклюш) Is a is a highly contagious disease typically characterized by a severe hacking cough followed by a high-pitched intake of breath that sounds like "whoop." The disease is of young infants, children, and adolescents. Despite an effective vaccine and generally high coverage with this vaccine, pertussis is one of the leading 12 causes of vaccine-preventable deaths worldwide in unvaccinated or incompletely vaccinated infants. ETIOLOGY and TRANSMITTION: Bordetella pertussis, a gram-negative bacterium releases exotoxin. Infection spreads via respiratory droplets or direct contact with nasal secretions. PATHOGENESIS: The most severe symptoms of whooping cough are caused by B. pertussis attaching itself to the respiratory tract cells with cilia. B. pertussis interferes with the normal function of the cilia (hair-like projections) to beat and sweep the respiratory tract clear of mucus, bacteria, viruses, dead cells, and other debris, and thus debris accumulates and triggers coughing by irritation of afferent fibers of n. Vagus and spreading of pathological impulses to the respiratory center activating cough reflex. Nearby centers are also involved: cough can be accompanied with vomiting, seizures, spasm of vessels. Classic symptoms include spasms (paroxysms) of uncontrollable coughing, followed by sharp, highpitched uptake of air, making the "whoop" sound that gave the disease its common name. There is no bacteremia! Exotoxin causes bronchospasm, generalized spasm on vessels, T-immunodeficient state. INCUBATION PERIOD is 4-21 days There are 3 stages of the disease: THE CATARRHAL stage lasts 1-2 weeks and is characterized by URT symptoms, low grade or absence of fever, mild cough and coryza. The patient is highly communicable during this stage and the first 3 weeks following it. THE PAROXYSMAL stage lasts 2- 6 weeks. Any stimulus (pain, light may promote cough with the specific pattern, described above. In the lungs emphysema develops. The child in the stage has remarkable symptoms: petecchia on the skin, the lungs, subconjuntival hemorrhage, cyanosis, eyelid edema, rupture of frenulum of the tongue. THE CONVALESCENT stage lasts 1-3 weeks. Cough reduces and disappears or can persist for several weeks. COMPLICATIONS are Pneumonia (90%), atelectasis, subcutaneous emphysema, intracerebral hemorrhage, nose bleeding, hemorrhage in the retina, formation of hernias, rupture of the diaphragm and drum membrane . DIPHTHERIA (Дифтерия) Diphtheria is an acute, toxin-mediated disease caused by the bacterium Corynebacterium diphtheriae. The name of the disease is derived from the Greek diphtheria, meaning leather hide. The disease was described in the 5th century BC by Hippocrates, and epidemics were described in the 6th century AD by Aetius. 13 The bacterium was first observed in diphtheritic membranes by Klebs in 1883 and cultivated by Löffler in 1884. Antitoxin was invented in the late 19th century, and toxoid was developed in the 1920s. ETIOLOGY and TRANSMISSION C. diphtheriae is an aerobic gram-positive bacillus. C. diphtheriae has three biotypes—gravis, intermedius and mitis. The most severe disease is associated with the gravis biotype, but any strain may produce toxin. All isolates of C. diphtheriae should be tested by the laboratory for toxigenicity. Toxigenicity occurs only when the bacillus itself is infected (lysogenized) by a specific virus (bacteriophage) carrying the genetic information for the toxin (toxic gene). Only toxigenic strains can cause severe disease. Exotoxin contains toxin A and toxin B. Toxin A causes cytotoxic effect due to the blockage of cytochrom B, toxin B helps toxin A penetrate into the cell. Also C. diphtheria produces neiroaminidase, hyalorunidase, necrotic factor. The source of infection is a sick person and carriers (vaccinated people carry up to 30 % bacteria, antitoxic immunity does not protect from bacteria carrying). People who do not have antitoxic immunity (not vaccinate children and adults with finished postvaccinal immunity) fall ill. The way of transmission is airborne and contact. The portal of infection is the mucous membrane of the upper airways, less often - damaged skin, conjunctiva and genitals. PATHOGENESIS In the portal of infection multiplication of the bacteria takes place, necrotoxin causes necrosis, slows down blood supply, exotoxin causes inflammation with severe increasing of permeability of vessels, releasing fibrin – that‘s why the hallmark of diphtheria is fibrinous inflammation with coats formation. On mucous membranes, lined with squamous epithelium there is the development of the diphteric type of fibrinous inflammation (films are firmly attached to the stroma), on mucous membranes, lined with single layered epithelium – croupous inflammation (films are easily detach). There is no bacteremia, but the absorption of exotoxins and products of dead tissue causes severe intoxication (toxinemia). The toxin is responsible for the major complications of myocarditis and neuritis and can also cause low platelet counts (thrombocytopenia) and protein in the urine (proteinuria), because in addition to epithelium of the URT Corynobacteria has tropism to: cardiac muscle; peripheral nervous system (nervous trunks, ganglions); adrenal glands; epithelium of proximal renal tubules; erythrocytes and leukocytes. CLASSIFICATION: For clinical purposes, it is convenient to classify diphtheria into a number of manifestations, depending on the site of disease: 14 1. 2. 3. 4. 5. 6. 7. Diphtheria of the pharynx (Pharyngeal and Tonsillar Diphtheria) -//- of the larynx (Laryngeal Diphtheria) -//- of the nose (Anterior Nasal Diphtheria) -//- of conjunctiva -//- the genitals -//- the skin (Cutaneous Diphtheria) -//- the ear INCUBATION PERIOD is about 2 - 10 days. Diphtheria of the pharynx. The most common sites of diphtheria infection are the pharynx and the tonsils. Infection at these sites is usually associated with a substantial systemic absorption of the toxin. The onset of pharyngitis is insidious. Early symptoms include malaise, sore throat, anorexia, and low-grade fever. Within 2–3 days, a bluish-white membrane forms and extends, varying in size from covering a small patch on the tonsils to covering most of the soft palate. There is a minimal amount of mucosal erythema surrounding the membrane. The membrane is adherent to the tissue, and forcible attempts to remove it cause bleeding (diphtheric inflammation). Patients with a severe form of the disease may develop a marked edema of the submandibular areas and the anterior neck along with lymphadenopathy, giving a characteristic ―bull neck” appearance. According to the level of subcutaneous edema there are subtoxic, toxic and hypertoxic (hemorrhagic and fulminant) forms. Diphtheria of the larynx is observed in less than 20 % of patients; usually it accompanies diphtheria of the pharynx; isolated forms are observed rarely. There is another type of fibrinous inflammation – croupous inflammation with the formation of grey films, freely attached to adjacent tissues. Detachment of the films with the obstruction of airways is called true croup resulting in asphyxia. Croupous inflammation extremely rarely goes down in the bronchi and bronchioles with the development of bronchopneumonia (descending croup). Usually intoxication is less marked. COMPLICATIONS Toxic shock (1-3 day) in case of toxic and hypertoxic forms it represents the first threshold of death. Cardiac complications: Acute cardiomyopathy (Myocardiodystrophy) - 4-8 day – usually transient and is followed by recovery. Myocarditis: early myocarditis is very dangerous. Death may occur on 12-17 day due to acute cardiac insufficiency - the second threshold of death. 15 Late myocarditis (2-3 week) –prognosis is better than for early myocarditis As an outcome of myocarditis diffuse cardiosclerosis may develop accompanied by Chronic Heart Failure Neurological complications are demyeliniziting in nature due to the blockage of synthesis of myelin in oligodendrocytes by exotoxin. Early polyneuritis (3-15 day) is the affection of n. glossopharyngeal and n. vagus: symptoms are disphagia, dysphonia Late polyneuritis (30-50 day) – paralysis of the extremities, muscles of the neck, but the most dangerous is paralysis of intercostal muscles and diaphragm and stoppage of breathing - the third threshold of death. Renal complications: necrotic nephrosis – acute renal insufficiency. Adrenal glands: dystrophic and necrotic changes, hemorrhages in adrenal glands with the development of acute suprarenal insufficiency. Asphyxia due to true croup. МENINGOCOCCAL INFECTION (Менингококковая инфекция) Meningococcal disease is an acute, potentially severe illness caused by bacterium Neisseria meningitidis. Disease believed to be meningococcal was first reported in the 16th century. The bacterium was first identified in the spinal fluid of patients by Weichselbaum in 1887. ETIOLOGY and TRANSMITTION: Neisseria meningitides is gram-negative diplococci, unstable in the environment. The outer membrane of the bacteria is surrounded by a polysaccharide capsule that is necessary for pathogenicity because it helps the bacteria resist phagocytosis and complement-mediated lysis. Neisseria meningitides produces endotoxin, hyaluronidase and neuraminidase. Endotoxin affects pre-capillaries, that blocks peripheral hemodynamics with the development of shock. Also endotoxin promotes DIC – syndrome. The source of the infection is a sick person or a carrier. The way of transmission is airborne. FORMS OF MENINGOCOCCAL DISEASE: Nasopharyngitis Purulent meningitis Meningococcemia PATHOGENESIS: Meningococci are transmitted by droplet aerosol or secretions from the nasopharynx of colonized individuals. The bacteria attach to and multiply on the mucosal cells of the nasopharynx. Penetration in the mucous membrane in 10 - 30 % cases can result in the development of acute nasopharyngitis. In a small proportion (less than 1%) of colonized individuals, the microorganism penetrates the mucosal cells and enters the bloodstream. The bacteria spread by way of the blood to many organs. In about 50%, mainly in children of early age, the 16 microorganism crosses the blood–brain barrier into the cerebrospinal fluid and causes purulent meningitis. An antecedent upper respiratory infection may be a contributing factor. In 0,1-1 % of cases meningococcemia (or meningococcal septicemia) results due to hematogenic dissemination of the bacteria. In some cases meningococcemia results as a complication of the purulent leptomeningitis. Acute nasopharyngitis Catarrhal inflammation of mucous membranes of the nose and throat with expressed hyperemia and edema of the posterior wall of the throat, serous or mucous exudates. The bacteriological research of smear from the pharynx is required for the diagnosis. Purulent leptomeningitis Purulent inflammation begins in the basal part of brain and distributes quickly to the convexital surface of the brain; the affected pia mater of the frontal, temporal and parietal lobes look like «a green cap» Frequently the purulent process involves the meninges of the spinal cord. Microscopically, neutrophils are revealed in the meninges. There is edema and focal inflammation extending to the cortex (encephalitis). Complications Purulent ependymitis and pyocephalus. Meningoencephalitis – spreading of purulent inflammation from pia mater to the tissues of the brain. The edema of the brain with dislocation of stem structures. Hydrocephalus which can arise after the development of exudate in the subarachnoid space with the blockage of the outflow of cerebrospinal fluid; further it results in atrophy of the brain parenchyma and accumulation of fluid in the cranial cavity. Meningococcemia It is a fulminant form of meningococcal infection. The duration is about 24 - 48 hours. It is a meningococcal sepsis with prominent endotoxinemia, resulting in endotoxic shock, accompanied by DIC- syndrome. Morphology: stellate hemorrhagic confluent rash, mainly on the skin of buttocks, lower extremities, eyelids and sclera. The rash begins from the lower extremities and is gradually distributed on the trunk and the upper extremities. Generalized damage of vessels of the microcirculation bed. 17 Necrosis with massive hemorrhages in adrenal glands with development of acute suprarenal insufficiency (Waterhouse-Friderichsen syndrome) severe uncontrollable hypotension acute renal insufficiency (necrotic nephrosis). Serous meningitis with hemorrhages in the pia mater. On the following scheme there is demonstration of the main hallmarks of different childhood infections. Measles 18 Rubella Chickenpox 19 Scarlet Fever Pic.1 Hallmarks of some childhood infections (from presentation of Dr. JOHN SRAGOWICZ PA-C, MPAS, 2008) 20 “It is easily to catch Tuberculosis, but it difficult to cure it ” Tuberculosis (TB) affected people for millennia. Roughly, one-third of the world's population has been infected with M. tuberculosis, and new infections occur at a rate of one per second. TB is the second most common cause of death from infectious disease (after HIV). Most of these deaths occur in developing countries. And nowadays TB starts to increase again after a period of decreasing. Mainly it is linked with an epidemic of HIV. ETIOLOGY: TB is caused by Mycobacterium tuberculosis (MBT) which is an acid fast bacillus (AFB). MBTs are approximately 1-10 m long, slender, rodshaped bacilli which may be curved or bent. These may be granular, isolated, in pairs or in groups. Ziehl-Nilsson stain is used for detection. Stained bacilli may present a beaded appearance. MBTs survive in droplets for 8 – 10 days, are relatively resistant to several chemicals including Phenol 5 % and Formaldehyde. Ethanol is suitable for application to superficial surfaces and skin gloves. Robert Koch (1843-1910) who got the Nobel Prize for Physiology and Medicine in 1905 is famous for the development of research techniques that are still applied by researchers throughout the world. Types of MBT that cause TB in humans: M. tuberculosis hominis M. tuberculosis bovis M. africanum M. avium intracellulare – TB in HIV-patients TRANSMISSION The source of infection is an open case of Pulmonary Tuberculosis. Every open case has a potential to infect 20 – 25 healthy people before they are cured or die. Only 1/10 of the infected will get the disease. Inhalation- M. tuberculosis may be expelled when an infectious person: coughs, sneezes, speaks, sings. IngestionM. bovis is transmitted with infected milk; selfswallowing of MBT with sputum leads to developing of tonsillar or intestinal TB) Inoculation - very rarely Transplacental- very rarely. 21 The probability that MTB will be transmitted depends on: Infectiousness of a person with TB disease Environment in which the exposure occurred Length of exposure Virulence (strength) of the tubercle bacilli Mycobacterium are killed at 600c in 15 – 20 mt. PECULARITIES OF INFECTION MBT does not produce toxins, allergy and immunity plays the major role. Cell-Mediated immunity plays a crucial role – reaction of hypersensitivity type 4 with the formation of granulomas; humoral immunity is not important. CD4 cell plays a role in immune mechanisms. They can actively produce Cytokines, Interferon-γ, which activate Macrophages to form Granulomas. PATHOGENESIS Macrophages are the primary cells infected by MBT. The bacteria enter macrophages by endocytosis. The glycolipid of bacterial cell wall blocks the fusion of the phagosome and lysosome. This is followed by bacterial multiplication inside the macrophages. Thus, the initial stage (<3 weeks) in a non-sensitized individual is characterized by bacterial multiplication in the pulmonary alveolar macrophages and airspaces, resulting in bacteremia and spreading to multiple sites in the body. After about 3 weeks of infection, TH1 cells, stimulated by MBT antigens, differentiate into mature TH1 cells by the action of IL-12. The mature TH1 cells in the lymph nodes and lungs produce IFN- , which stimulates formation of phagolysosome in infected macrophages, and causes nitric oxide induced oxidative damage to the cell wall and DNA of the mycobacteria. Activated macrophages, stimulated by IFN- , produce TNF and recruit monocytes, they differentiate into cells with pale cytoplasm, eosinophilic nuclei, elongated and vesicular - “Epithelioid Cells‖. Some macrophages instead of Epithelioid cells fuse to form Langhans cells (multinucleated cells with about 20 nuclei, nuclei are arranged like a horse-shoe or like a ring). Next type of cells – foreign-body Giant cells - can be formed due to increasing the amount of nuclei (100 nuclei, arranged in the center of a cell or distributed in the ytoplasm). The immune response is usually accompanied by hypersensitivity and tissue destruction - Caseous necrosis. So, the structure of TB Granuloma is: In the center- caseous necrosis 22 Layer of macrophages Layer of epithelioid cells Langhans cells Giant cells Lymphocytes Fibrosis. BUT! The presence of these layers shows the pathologist the duration of this process and stage (activation or healing stage). All these layers can be seen in healing of a TB granuloma, in the acute period we can see only the presence of caseous necrosis with macrophages, epitheliod cells and lymphocytes. Hallmark of Healing ≈ “Productive‖ or ―Proliferative‖ reaction: activation of cells and formation of fibrosis. Hallmark of Activation ≈ ―Exudative reactions‖ - caseation and softening of the foci. Latent TB Infection (LTBI) Occurs when MBT are in the body, but the immune system is keeping them under control. LTBI is invisible by means of X-ray and can be detected only with the Mantoux tuberculin skin test (TST). A positive tuberculin test result signifies cellmediated hypersensitivity to tubercular antigens but does not differentiate between infection and disease. People with LTBI are NOT infectious. But in any time LTB may transform to TB disease. TB Disease Develops when immune system cannot keep MBT under control. The disease can develop very soon after infection or many years after infection. About 10% of all people with normal immune systems who have LTBI will develop TB disease at some point in their lives. However, certain clinical condition can increase the risk of tuberculosis like diabetes mellitus, Hodgkin‘s lymphoma, chronic lung disease (particularly silicosis), chronic renal failure, malnutrition, alcoholism and immunosuppression. People with TB disease are often infectious, especially in case of ―open forms‖. Classification of TB Primary TB Post-primary (Secondary) TB PRIMARY TUBERCULOSIS - the first infection with the tubercle bacillus is known as primary tuberculosis. The disease develops in a previously unexposed and unsensitized individual. Morphology: Primary tuberculosis almost always begins in the lungs. In case of infecting via the alimentary tract MBT may implant in tonsils or ileoceacal region. Primary TB of the skin (due to inoculation) is very rare. Typically, the inhaled 23 bacilli implant in the distal airspaces of the lower part of the upper lobe or the upper part of the lower lobe (III, VIII, IX, X segments) due to most of the inspired air being distributed here and form a subpleural lesion- primary affect. Primary affect is the focus of exudative inflammation with quick development of the caseous necrosis in the center and perifocal inflammation. Usually, it has the size from alveoli to segmentum. The pleura is also involved with the formation of fibrinous or sero-fibrinous pleurisy. This subpleural lesion (primary affect) along with involving the draining lymphatics (lymphangitis) and the lymph nodes (lymphadenitis) is called the primary tuberculous complex (Ghon’s complex). Lymphangitis is characterized by lymphostasis and numerous tubercles along the lymphatic vessel. It looks like a ―road‖ from primary affect to hillary lymph nodes. Lymphadenitis is characterized by bifurcational lymph nodes increase in size; it has a grey cut surface due to the presence of caseous necrosis. The same structures are distinguished in case of extrapulmonary localization of primary TB. Outcomes of primary TB 1. HEALING – due to activation of the immune system, exudative reactions change to productive, a tuberculous granuloma is surrounded with fibrosis, petrification and ossification may take place with the formation of Ghon‘s focus. The same process – scaring with petrification – occurs in affected lymph nodes. 2. CHRONISATION OF PRIMARY TB (―chronic tuberculous intoxication‖) develops after healing of the primary lesion but the process progresses in lymph nodes. It is characterized by micropoliadenitis and the so-called ―paraspecific reactions‖: erythema nodosum, blepharitis, rhinitis and conjunctivitis. 3. PROGRESSION OF PRIMARY TB: - Hematogenic dissemination – MBT from primary lesion or lymphadenitis passes into the blood and spreads to different organs with the formation of tuberculi (granulomas) in them. According to the size of this foci milliary (1 mm) and large focal (0,5 sm) forms are distinguished. The most dangerous is dissemination to meningitis. Dissemination in the apex of the lungs in this case has a specific name – Simon’s foci. - Lymphogenic dissemination – involving of new groups of lymph nodes with the formation of caseous necrosis (subclavicular, bronchial and paratracheal) - Growth of primary lesion – spreading of caseous necrosis leads to involvement of the total lobe – primary caseous pneumonia (―galloping consumption‖) or necrosis may be drained via bronchi with the formation of primary caverna (cavity) and primary cavernous TB. SECONDARY TUBERCULOSIS (post-primary) tuberculosis – Secondary tuberculosis is the pattern of the disease that arises in a previously sensitized host. 24 It usually results from a reactivation of latent primary lesion after many years of an initial infection, particularly when host immunity is decreased or uncommonly may follow primary tuberculosis, or reinfection from the environment. Peculiarities of secondary TB: The preexistence of hypersensitivity contributes to an immediate and marked tissue response. Cavitation is frequent, leads to spreading of bacilli during coughing. The regional lymph nodes are less prominently involved in secondary tuberculosis. Secondary pulmonary tuberculosis is classically localized to the apex of the upper lobes of the lung (right lung is more commonly affected as compared to the left one because of high oxygen tension in the apices). Progressive pulmonary tuberculosis is observed in the elderly and the immunosuppressed individuals. The apical lesion enlarges with the increase in the area of caseation. The erosion of blood vessels (particularly bronchial artery) can result in hemoptysis. Pleural effusion or empyema in revealed the pleural cavity. If the treatment is adequate, the disease can be controlled, but if it is inadequate, the infection may disseminate through airways, lymphatics or the vascular system. Histologically, the active lesion shows characteristic tubercles, composed of Epithelioid cells and Langhan‘s cells with central caseation. The lesion of secondary pulmonary tuberculosis can heal with fibrosis either by itself or after therapy, or it can progress along the following several different pathways. Forms of secondary TB correspond to the stages of progression or healing of the disease . FORMS of SECONDARY TB: 1. Acute local 2. Fibro-local 3. Infiltrating 4. Tuberculoma 5. Caseous pneumonia 6. Acute cavernous 7. Fibro-cavernous 8. Cirrhotic 25 ACUTE FOCAL TB AND FIBRO-FOCAL TB Acute focal TB usually affects people of 20-25 years of age One or few small foci (not more than 2 cm) in the apex (I, II segments) of the right, or less commonly, left lung (―Abricosov’s foci” of reinfection) Exudative reaction prevails. In the course of treatment or spontaneous tissue reaction changes to proliferative, the area of necrosis undergoes incapsulation and transforms to Fibro-focal TB (“Ashoff- Pulle focus”). INFILTRATIVE TB Usually affects young people The form develops due to the progression of acute focal TB; exacerbation of fibro-focal; lymphogenic spead of MBT from lymph nodes. Main location is upper lobes. The affected part is endured, the cut section is yellowish-grey, granular, with small foci of caseous necrosis in the center. The focus of 3-5 cm in diameter, connected with the hilum via inflamed bronchus and having a ―tennis racket‖ appearance, is called Assmann-Redecker's infiltrate. Exudative reaction is prominent, perifocal inflammation is remarkable, caseous necrosis is not prominent Types of Infiltrative TB are subdivided depending on their extension and degree of exudative tissue reaction: Rounded infiltrate (involves segment or lobule) Cloud-like infiltrate (few lobules) Pericessuritis (affection a part of the lobe near interlobular fissure with inflammation of the pleura) Lobar infiltrate (lobe) OUTCOMES: Progression to caseous pneumonia and cavernous TB or healing with the reduction of exudative reaction with the formation of fibro-focal TB or tuberculoma (if the focus is small) or cirrhotic TB with severe deformation of the lung (in case of a large focus - lobar infiltrate). CASEOUS PNEUMONIA The disease usually develops in patients with severe immunosuppression. It is characterized by the prevalence of caseous necrosis with the lack of cellular reaction, acute progressive course and early formation of the cavities, complicated with bleeding. usually involves a lobe or the whole lung 26 Macroscopically: usually starts form the upper lobe, the lung is dense and granular; it has a yellowish-grey color. Pleurisy is common. Drainage of caseous necrosis through the bronchi causes the formation of cavities with the transformation to cavernous TB. OUTCOME and COMPLICATIONS: the disease is usually fatal due to severe intoxication and respiratory insufficiency CAVERNOUS TB This is a progression of other forms of secondary TB (primary cavernous TB can also a variant of progression of primary TB!) Activation of depletion of bacteria due to a decrease of immunity or increase of sensibilization, joining of secondary infiltration by PNLs, production of proteolytic enzymes with the lysis of tissue promotes the formation of the cavity. Draining of these masses through the bronchus cause the formation of a cavity. The usual locations of cavities – 1-3 or 6 segmentum (where other forms of secondary TB are located more commonly). Caverns may be small (<2 cm), middle-sized (2-4 cm), large (4-6 cm), giant (>6 cm). Acute pneumoniogenic cavern consists of 2 layers: internal layer of necrosis with abundant neutrophilic infiltration, and a zone of specific tubercular inflammation with few Epitheliod, Langhans cells and lymphocytes. OUTCOMES: collapse of the cavity with scarring (formation of the ―stellatum scar‖) or progression to fibro-cavernous TB COMPLICATIONS: bleeding, pneumothorax in case of rupture of the subpleural cavity. FIBRO- CAVERNOUS TB It is the progression of acute cavernous TB with the formation of fibrosis around the wall of the cavity. Localization and size are the same in acute cavernous TB, but the wall of a cavern consist of 3 layers: internal – caseous necrosis with PNLs, middle- specific inflammatory reactions with Epitheliod, Langhans‘ and giant cells; external layer - non-specific fibrous tissue. Usually an old cavern is connected with the bronchi and the affected lobe is contracted due to massive fibrosis. Bronchogenic spreading of MBT to the lower lobe or another lung is common! COMPLICATIONS: 27 1. Bleeding 2. Chronic respiratory insufficiency with the formation of chronic corpulmonale and right ventricle insufficiency 3. Pneumothorax 4. Amyloidosis of the kidney with chronic renal failure 5. Empyema of the pleura 6. Pleural adhesions TUBERCULOMA The focus of caseous necrosis is more than 1 cm, surrounded by a fibrous capsule. Usually it is healed infiltrative TB or large-focal hematogenic TB. Usually localization is in the I or II segments of the lung. They may be solitary and multiple Classification is based on the size and is similar to the classification of caverns. A tuberculoma may be stationary (no symptoms), regressing (decreasing in size, draining with collapse)) and progressing (increasing of infiltration in the periphery, rupture of the wall, bronchogenic dissemination) It can develop into caseous pneumonia (due to exacerbation and spreading of caseous necrosis) or fibro-cavernous TB (after draining of the caseous necrosis, but with the remaining capsule) TYPES of tuberculoma: Solitary tuberculoma – round structure with caseous necrosis in the center, surrounded by dense fibrous tissue. The capsule has no vessels- it is no use to treat it therapeutically. Conglomeratic – consists of several caseous foci combined by a nonuniform fibrous capsule. Its contours are irregular. Laminated tuberculoma – concentric arrangement of caseous necrosis, altering with fibrosis (in case of slow progression). CIRRHOTIC TB Massive formation of connective tissue with symptoms of active TB process. It takes place as a prolonged course of disseminated pulmonary TB, healing of infiltrative TB (lobitis), fibro-cavernous TB. Beside the morphological picture of pneumosclerosis, compensatory emphysema and bronchoectasis develop in the remaining areas. 28 Massive pleural adhesions with increasing of thickness of the pleura. COMPLICATIONS are similar to fibro-cavernous TB DISSEMINATED TB Dissemination – is a complication of primary as well as any form of secondary TB in case of progression and destruction of the focus. Dissemination may be hematogenic, lymphogenic and bronchogenic. According to its genesis disseminated tuberculosis can be classified into primary and post-primary. Primary disseminated tuberculosis can develop immediately after bacterial invasion into the body and usually spreads from tubercular foci in incompletely calcified intrathorasic lymph. Mycobacteria in this case spread through lymph vessels and blood. The development of post-primary disseminated tuberculosis is connected with sources of bacteremia. M. tuberculosis spread out of the organs into other body organs and tissues through lymph and blood (this is the most frequent way) or bronchi. According to the course of the dissemination there are 3 types: 1. Acute miliary tuberculosis - the term miliary describes the resemblance of the pathologic lesions to millet seeds. This form of tuberculosis is characterized by the bilateral symmetric micronodular monomorphic dissemination with subpleural location, which spreads up to down. There is a possibility of the extrapulmonary location. Small (1-3 mm) bilateral symmetric multinodular shadows appear in 1014 days. First they are composed only of caseous necrosis, then specific inflammatory infiltration appears. The shadows on X-rays films are monomorphic and have the small intensity. The general condition of the patient in case of acute tuberculosis is generally severe. In case of proper treatment the prognosis is good: the dissemination dissolves without any abnormalities in 6-9 months after the onset of the disease. If the therapy starts in the advanced stage, the fatal outcome can occur in 3-4 weeks from the onset of the disease. 2. Subacute dissemination - is characterized by the bilateral polymorphic multinodular dissemination. It is not always symmetric and spreads from upper lobes downwards. There are often extrapulmonary locations of the lesion (mainly the larynx and serous membranes, and also lymph nodes, bone, skin, eyes and other organs). The disease begins subacutely. Its course is wavelike. In case of inadequate treatment the disease actively progresses. X-ray examination shows growth and fusion of the lung foci and then the formation of new cavities. In some cases subacute disseminated TB results in caseous pneumonia and fatal outcome (in 5-6 months). In case of adequate and early treatment the prognosis is favorable 29 as a rule. The TB foci can resolve completely, the cavities heal. But usually there are residual changes: pneumosclerosis, calcified foci, pleural layers and emphysema. 3. Chronic dissemination - is characterized by non-symmetrical bilateral polymorphic multinodular dissemination. Extrapulmonary localization is common. Chronic dissemination is the most common clinical form of disseminated tuberculosis in adults. This disease has a wavelike course; it is connected with the appearance and disappearance of new tuberculous foci. All 3 ways of dissemination takes place: lymphogenous, bronchogenous and hematogenous. Polymorphic tuberculous foci of different form, size and density are detected on Xray films. They are mainly localized in the subpleural area and can join together. Their size decreases, but the distance between them increases from upper to lower lobes. The cavities have an irregular form and infiltration around them. Fibrotic changes, pleural adhesions and emphysema accompany the disease with the formation of cor-pulmonale. According to the organs, affected by dissemination: 1. Generalized disseminated TB 2. Disseminated TB with predominant affection of the lungs 3. Disseminated TB with predominate extra-pulmonary affections Generalized haematogenic TB is characterized by development of many tubercles in all internal organs. Many foci of caseous necrosis without any signs of proliferative reaction are seen (so-called Landouzy‘s sepsis). Also generalized TB may have a chronic course – proliferative reactions dominates in disseminated foci in this case. Disseminated TB with predominant affection of the lungs Affection may spread via blood stream and via bronchi – to another lung or lower lobes of the same lung. In case of haematogenic dissemination, apices of the lungs are affected more commonly (due to better blood supply), lower lobes are affected due to gravity. According to size of foci this type can be: 1. Acute milliary TB – 1 mm in size, exudative reaction prevails, formation of foci with necrosis follows cavitation. The term miliary tuberculosis is used to describe the resemblance of the pathologic lesions to millet seeds. This form of tuberculosis is characterized by the bilateral symmetric micronodular monomorphic dissemination with subpleural location, which spreads up to down. 2. Large focal TB – foci 0.5-1.0 sm, proliferative reaction with developing of fibrosis prevails (subacute dissemination), bronchoectasis and finally corpulmonale develops 30 Disseminated TB with predominate extra-pulmonary affections Most commonly affected organs are: 1. Nervous system (TB meningitis, meningoencephalitis, affection of spinal cord). TB MENINGITIS is a usual complication of primary TB; also may follow milliary TB. May spread via CSF from focus in brain, located after bacteremia in primary TB. Macroscopically: the brain is covered by greeenish exudate, jelatinous, especially on the base of the brain + numerous tubercles on meningies are visible. 2. Genito-urinary system (kidneys, urinary bladder, fallopian tubes, endometrium, ovary, prostate, testis). Tuberculous salpingitis – is the most common reason for infertility if developing countries. 3. Bones and joints – often in children: The major sites of osseous and articular tuberculous lesions include the epiphyses of the long bone shafts, bodies of the short bones: f.e. vertebrae and diaphyses of the digital phalanges. Analogically, this type of tuberculosis falls into the major forms: tuberculous spondylitis, or spinal TB (40%). Tuberculous spondylities is complicated with cold abscess in the soft tissues around spinal column. tuberculous coxitis, or hip jojnt TB (20%); tuberculous gonitis, or knee joint TB (15- 20%) Pathogenesis of bones and joint lesions involves the three stages: stage 1 – pre-arthritis- formation of the bone focus in the epiphysis around the joint; stage 2 - arthritis - extension of the process to the joint with resultant secondary arthritis; stage 3 - post-arthritis- stabilization of the disease with the evidence of its complications. SYPHILLIS (СИФИЛИС) Syphilis, which is also called lues (from a Latin word meaning "plague"), has been a major public health problem since the sixteenth century. The disease was treated with mercury or other ineffective remedies until World War I, when effective treatments based on arsenic or bismuth were introduced. These were succeeded by antibiotics. ETIOILOGY and TRANSMISSION: Syphilis is caused by a spirochete, Treponema pallidum. A spirochete is a thin spiral- or coil-shaped bacterium that enters the body through the mucous membranes or breaks in the skin. In 90% of cases, the spirochete is transmitted by sexual contact. Transmission by blood transfusion is possible but rare, not only because blood products are screened for the disease, but also because the spirochetes die within 24 hours in 31 stored blood. Other methods of transmission are highly unlikely because T. pallidum is easily killed by heat and drying. Transmittion by contacts is also possible. Syphilis may be either congenital or acquired. Acquired syphilis has three stages (primary, secondary, and tertiary). Transplacental transmition is possible. The risk for transplacental transmittion is the highest in 4-5 month of pregnancy. INCUBATION PREIOD: from 10–90 days AQUARED SYPHILIS Primary syphilis The primary lesion develops at the site of infection, which heals in 2-6 weeks. The small, painless papule rapidly forms an ulcer (the chancre). The chancre is usually single, round or oval, painless, surrounded by a bright red margin, indurated with a clean base, and discharging clear serum. Chancres may be atypical - eg, multiple, painful, purulent, destructive and may be extragenital. They are usually found in heterosexual men on the coronary sulcus, the glans and inner surface of the prepuce but may appear on the shaft and beyond. In homosexual men, they are usually found in the anal canal and, less frequently, in the mouth and genitalia. In women, they are found on the vulva, labia and, much less frequently, on the cervix. Extragenital sites are the lips, mouth, buttocks and fingers. There are enlarged regional lymph nodes that are painless, discrete, firm and not fixed to surrounding tissues. Secondary syphilis Secondary syphilis often appears 6 weeks after the beginning of the primary lesion but may overlap or not appear for several months. Multisystem involvement occurs within the first 2 years of infection. Systemic symptoms are mild or absent, but include nighttime headaches, malaise, slight fever and aches. A generalized polymorphic rash often affects the palms, soles and face. The rash is classically non-itchy; however, it may be itchy, especially in darkskinned patients. It is associated with generalised painless lymphadenopathy. Papules enlarge into condylomata lata (pink or grey discs) in moist warm areas. Papule lesions disappear spontaneously. There may also be mucocutaneous lesions. 32 Less common presentations include patchy alopecia, anterior uveitis, meningitis, cranial nerve palsies, hepatitis, splenomegaly, periostitis and glomerulonephritis. In 80% of cases, patients enter the latent asymptomatic stage which for over half of them persists for life. In about 20% of patients, an infectious relapse occurs during the next year. Tertiary syphilis Untreated syphilis progresses to a third or tertiary stage in about 35–40% of patients (only those who go untreated). Patients with tertiary syphilis cannot infect others with the disease. It is thought that the symptoms of this stage are a delayed immune hypersensitivity reaction to the spirochetes. This consists of three major clinical manifestations, which may coexist: Neurological syphilis: 1. Asymptomatic. In this form of neurosyphilis, the patient's spinal fluid gives abnormal test results but there are no symptoms affecting the central nervous system. 2. Meningovascular. This type of neurosyphilis is marked by changes in the blood vessels of the brain or inflammation of the meninges (the tissue layers covering the brain and spinal cord). The patient develops headaches, irritability, and visual problems. If the spinal cord is involved, the patient may experience weakness of the shoulder and upper arm muscles. 3. Tabes dorsalis. Tabes dorsalis is a progressive degeneration of the spinal cord and nerve roots. Patients lose their sense of perception of body position and orientation in space (proprioception), resulting in difficulties walking and loss of muscle reflexes. They may also have shooting pains in the legs and periodic episodes of pain in the abdomen, throat, bladder, or rectum. Tabes dorsalis is sometimes called locomotor ataxia. 4. General paresis. General paresis refers to the effects of neurosyphilis on the cortex of the brain. The patient has a slow but progressive loss of memory, decreased ability to concentrate, and less interest in self-care. Personality changes may include irresponsible behavior, depression , delusions of grandeur, or complete psychosis. General paresis is sometimes called dementia paralytica, and is most common in patients over 40. Cardiovascular syphilis: Cardiovascular syphilis occurs in 10–15% of patients who have progressed to tertiary syphilis. It develops between 10 and 25 years after infection and often occurs together with neurosyphilis. Cardiovascular syphilis usually begins as an 33 inflammation of the arteries leading from the heart and heart attacks, scarring of the aortic valves, congestive heart failure, or the formation of an aortic aneurysm. Gummata: Gummas are rubbery tumor-like growths that are most likely to involve the skin or long bones but may also develop in the eyes, mucous membranes, throat, liver, or stomach lining. Morphologicaly – gumma is granuloma. The specific feature of the granuloma is a presence of eosinophills. Gummas are increasingly uncommon since the introduction of antibiotics for treating syphilis. CONGENITAL SYPHILIS Congenital syphilis is syphilis present in the antenatal and intranatal period, and occurs when a child is born form a mother with secondary syphilis. Syphilis can cause miscarriages, premature births, stillbirths, or death of newborn babies. Some infants with congenital syphilis have symptoms at birth, but most develop symptoms later. Classification Early congenital syphilis This is a subset of cases of congenital syphilis. Newborns may be asymptomatic and are only identified on routine prenatal screening. If not identified and treated, these newborns develop poor feeding and rhinorrhea. By definition, early congenital syphilis occurs in children between 0 and 2 years old. After, they can develop late congenital syphilis. Symptomatic newborns, if not stillborn, are born premature, with hepatosplenomegaly, skeletal abnormalities, pneumonia and a bullous skin disease known as pemphigus syphiliticus. Late congenital syphilis By definition, it occurs in children at or over 2 years of age who acquired the infection transplacentally. Symptoms include blunted upper incisor teeth - Hutchinson's teeth inflammation of the cornea known as interstitial keratitis deafness from auditory nerve disease frontal bossing (prominence of the brow ridge) saddle nose (collapse of the bony part of nose) hard palate defect swollen knees saber shins short maxillae 34 protruding mandible A frequently-found group of symptoms is Hutchinson's triad, which consists of Hutchinson's teeth (notched incisors), keratitis and deafness and occurs in 63% of cases. Treatment (with penicillin) before the development of late symptoms is essential. TYPHOID FEVER (БРЮШНОЙ ТИФ) The WHO identifies typhoid as a serious public health problem. Its incidence is highest in children and young adults between 5 and 19 years old. This fever received various names, such as gastric fever, abdominal typhus, infantile remittent fever, slow fever, nervous fever, pathogenic fever, etc. ETIOLOGY and TRANSMITTION: the diseases is transmitted by the ingestion of food or water contaminated with the feces of an infected person, which contain the bacterium Salmonella enterica, serovar Typhi. The organism is a Gram-negative short bacillus that is motile due to its peritrichous flagella. Not only ill persons, but also asymptomatic carriers are potentially infecting. PATHOGENESIS The pathophysiology of typhoid fever is a complex process which proceeds through several stages. The disease begins with an asymptomatic incubation period of 7-14 days, (inversely related to the size of the infecting dose), during which bacteria invade macrophages and spread throughout the reticuloendothelial system. The first week of symptomatic disease is characterized by progressive elevation of the temperature followed by bacteremia. The second week begins with the development of rose spots, abdominal pain and splenomegaly. The third week is marked by a more intense intestinal inflammatory response particularly in the Peyer‘s patches with associated necrosis which can result in perforation and hemorrhage. These clinical stages are associated with complex cellular events just now being understood. First, ingested bacteria must survive the acidic environment of the stomach. The known increased risk of typhoid fever with concomitant Helicobacter pylori infection may express itself via the hypochlorhydria associated with chronic H.pylori infection. Invading organisms pass through the intestinal epithelial cells 35 and come into contact with phagocytic cells in the Peyer‘s patches of the intestinal wall. However the macrophages do not kill the bacteria. Thence, bacterial replication is primarily intracellular. Salmonella avoids encapsulation in lysosomes by diverting normal cellular mechanisms. Bacteria inject effector proteins into the cells of the innate immune system (macrophages and natural killer cells) though a type III protein secretion system (TTSS) which stimulate both pro and antiinflammatory responses. Over the asymptomatic incubation period of 7-14 days the bacteria proliferate and spread through the blood stream to other cells in the reticuloendothelial system in the liver, spleen, bone marrow and gall bladder. As replication inside phagocytic cells continues, bacteria are shed into the blood stream in sustained but low concentrations and the clinical syndrome of fever, headache and abdominal pain begins. The gallbladder is felt to be a significant site for ongoing exposure of intestinal epithelial cells to the pathogen. The inflammatory response to this process of repeated exposure is felt to give rise to the necrosis which is a prominent feature of the disease. This occurs in areas of greatest macrophage concentration such as the Peyer‘s patches and explains why intestinal bleeding and perforation are the most frequent complications. Elsewhere typhoid nodules, foci of macrophages and lymphocytes proliferate. As the infection progresses the typical changes of sepsis accumulate in the heart, brain and kidneys. If not interrupted this process may lead to circulatory failure and death from overwhelming sepsis. The classic disease description includes bacteremia and fever during the first week, as well as nonspecific symptoms as chills, headache, anorexia, sore throat, myalgia, psychosis and mental confusion in 5 to 10% (typhoid state). A coated tongue, tender abdomen, hepatomegaly, and splenomegaly are common. In the second week, a few rose spots, blanching erythematous maculopapular lesions, approximately 2 to 4 mm in diameter appear in 5 to 30 percent of cases. They usually occur on the abdomen and chest and more rarely on the back, arms, and legs. In some patients green yellow liquid "pea soup" diarrhea may occur. A relative bradycardia in relation to fever, intestinal constipation, and diarrhea in smaller number of patients (mainly in young children and adults with HIV infection) may occur. This classic pattern of typhoid fever has been modified by earlier diagnosis and more effective treatment. In spite of these advances, some patients still may present the clinical manifestations of first and second weeks. In the cases without treatment or correct diagnosis, the typhoid fever prolongs to third week. In this period, the inflammatory lesions are intense in Peyer's patches and intestinal lamina propria (with abundant monocytes, macrophages and lymphocytes). There is lymphoid hyperplasia in the ileocecal area followed by 36 ulceration and necrosis, with subsequent gastrointestinal bleeding or intestinal perforation. Symptoms as anorexia, weight loss, intense weakness, mental confusion, and others, may occur in typhoid fever especially when there is a delay in the diagnosis and treatment. Pathological features: The earliest pathologic changes are in the stages of bacterial attachment and penetration. Bacteria are firmly attached to intestinal epithelium with an accompanying degeneration of the brush borders. Later as the salmonellae pass to lymphoid follicles of the intestine, diffuse enterocolitis and hypertrophy of Peyer‘s patches develops. This is followed by necrosis of intestinal and mesenteric lymphoid tissues, focal granulomas in the liver and spleen, and characteristic mononuclear inflammatory cells (―typhoid nodules‖) in many organs. Typhoid nodules are primarily aggregates of altered macrophages (―typhoid cells‖) that phagocytose bacteria, erythrocytes and degenerated lymphocytes. These nodules also contain plasma cells and lymphocytes, but not typically neutrophils – so, granuloma is a morphological substrate of typhoid fever. The most common sites for typhoid nodules are the intestine, mesenteric lymph nodes, spleen, liver, and bone marrow. Less commonly, the kidney, testes, and parotid gland are affected. Although the pathologic changes of typhoid fever may not correlate precisely with the clinical stages, certain patterns are characteristic. During the incubation stage there is a mild enteritis, mesenteric lymphadenitis, and hyperplasia of intestinal lymphoid tissue, primarily of Peyer‘s patches of the ileum and solitary lymphoid follicles of the cecum. The lymphoid hyperplasia may resolve or may progress to capillary thrombosis. Thrombosis causes the adjoining intestinal mucosa to enlarge during the phase of active invasion and then become necrotic. This process gives rise to the characteristic lesions, which are elevated 0.1 to 0.4 cm above adjacent mucosa. While bacilli continue to proliferate, dying bacilli release endotoxins that cause toxemia. The necrotic mucosa desquamates, producing ulcers that conform to Peyer‘s patches and are concentrated along the antimesenteric border. The ulcers may bleed or perforate, usually during lysis. Most perforations are near the ileocecal valve, measure less than 1 cm across, and lead to peritonitis. Interestingly, these areas become repopulated with lymphoid cells and heal without scarring. During active invasion the mesenteric lymph nodes enlarge and develop typhoid nodules, focal hemorrhages, and necrosis, changes which resemble those in the intestinal lymphoid tissue. The spleen becomes large and hyperemic and microscopically shows typhoid nodules in the red pulp. The hyperplastic white 37 pulp exhibits areas of focal necrosis. The enlarged liver displays sinusoids lined with swollen Kupffer‘s cells and histiocytes. Focal necrosis of liver cells is common. During convalescence, the intestine returns to normal, with minimal scarring of the mucosa. Adhesions are rare. Typhoid nodules in various organs are resorbed without distortion of the architecture. The capsule of the spleen, however, may become fibrotic, giving the appearance of ―sugar coating‖. Skeletal muscles regenerates and toxic changes of heart disappear. Stages: every stage lasts for 1 week 1. Medullary swelling: swelling of lymphoid follicles in ileum and infiltration by macrophages 2. Necrotic stage: necrosis occurs in lymphoid follicles 3. Ulceration stage: shedding of necrotic tissue results in ulcer formation with bleeding and perforation 4. Healing: no scars after ulcer healing Complications Complications occur in 10-15% of patients, particularly those who have been ill for more than 2 weeks. Gastrointestinal hemorrhage, perforation and encephalopathy are the most important. GI hemorrhage is most common but usually resolves without surgery. Single perforations are most common (70%) and in the terminal ileum, but multiple perforations may occur. The most important surgical complications are: hepatic or splenic abscess, splenic rupture and pancreatitis. Encephalomyelitis, osteomyelitis, glomerulonephritis and renal failure may also occur. Myocarditis is a common cause of circulatory collapse. Toxemia may cause other complications, including ileus; mild fatty liver; a flabby heart with dilated ventricles, vacuolization of cardiac myocytes, and cardiac arrhythmia that may cause sudden death; mild interstitial pneumonitis; swelling and degeneration of the proximal tubular epithelium of kidney; ―ring‖ hemorrhages in brain; capillary microthrombi; and degeneration of skeletal muscles. CHOLERA (ХОЛЕРА) Cholera is one of the most dramatic of the water-borne diseases. It has affected the greater part of India in both recent years and centuries ago. ETIOLOGY: etiological agent is Vibrio cholerae. According to their biological properties V. cholerae is divided into 2 biovars: V. cholerae classical, V. cholerae El-Tor. The classical and El Tor vibrios share the same O-Ag and is agglutinated by O1antiserum (O-1 serogroup). 38 According to structure of the O1-Ag species V. cholerae is subdivided into 3 serotypes: Ogawa (AB), Inaba (AC), Hikojima (ABC). Factors of virulence. 1. Exotoxin (choleragen, cholera enterotoxin, cholera toxin, CT, or CTX). The toxin molecule, consists of one A and B subunits. The B (binding) units attach to the ganglioside receptors on the surface of jejunal epithelial cells. The A (active) unite causes prolonged activation of cellular adenylate cyclate and accumulation of cAMP, leading to outpouring into the small intestinal lumen, of large quantities of water and electrolytes and the consequent watery diarrhea. 2. Endotoxin. Cholera vibrios also possess the lipopolysaccharide O antigen (LPS, endotoxin), as in Gram negative intestinal bacilli. This apparently plays no role in the pathogenesis of cholera but is responsible for the immunity induced by killed vaccines. 3. Adherence factors (pili). 4. Proteolytic enzymes (gelatinase, mucinase) EPIDEMIOLOGY AND PATHOGENESIS Cholera is an exclusively human disease. Infection is acquired through fecally contaminated water or food. Direct person-to-person spread by contact may not be common but hand contamination of stored drinking water has been shown to be an important method of domestic spread of infection. In the small intestine, vibrios are enabled to cross the protective layer of mucus and reach the epithelial cells by chemotaxis, motility, mucinase and other proteolytic enzymes. Adhesion to the epithelial surface and colonisation may be facilitated by special fimbria such as the 'toxin co-regulated pilus'. The massive loss of water and electrolytes by action of enterotoxin, leads to 1. dehydration causing hemoconcentration, anuria and hypovolaemic shock 2.base-deficience acidosis 3. muscle cramps due to hypokalaemia. Stages: INCUBATION (10–14 days) 1. Stage of enteritis which is the inflammation of the small intestine which diarrhea. It lasts up to 24 to 48 hours. The main symptoms are profuse painless diarrhea (characteristically called ―rice water stool‖). The diarrhea could have fishy odor; also the patients may lose up to ten to twenty liters of diarrhea in a day. 2. Stage of gastroenteritis which involved spreading of the inflammation to both the stomach and intestines. In this stage the patients have profuse diarrhea, continuous vomiting leading to dehydration, and decrease of body temperature, urine and electrolytes. All these could lead to appearance of convulsions. 39 3. Algid period (algid means ―cold‖). There is a resultant wrinkling of the skin followed by cyanosis; voice becomes hoarse sometimes lost totally. Complications: Low blood sugar (hypoglycemia). Dangerously low levels of blood sugar (glucose) — the body's main energy source — may occur when people become too ill to eat. Children are at greatest risk of this complication, which can cause seizures, unconsciousness and even death. Low potassium levels (hypokalemia). People with cholera lose large quantities of minerals, including potassium, in their stools. Very low potassium levels interfere with heart and nerve function and are life-threatening. Kidney (renal) failure. When the kidneys lose their filtering ability, excess amounts of fluids, some electrolytes and wastes build up in your body — a potentially life-threatening condition. In people with cholera, kidney failure often accompanies shock. DYSENTERY (Дизентерия) Dysentery is an inflammation of the intestine causing diarrhea with blood. Other symptoms may include fever, abdominal pain, and rectal tenesmus (a feeling of incomplete defecation). It is caused by a number of types of infection such as bacteria, viruses, parasitic worms, or protozoa. It is a type of gastroenteritis. The mechanism is an inflammatory disorder of the intestine, especially of the colon. Shigellosis, or bacillary dysentery, is an intestinal infection, especially of the colon, that is a major public health problem in many undeveloped and developing countries, and is the main cause of childhood diarrhea. The disease is characterized by the painful and frequent passage of stools that contain large amounts of blood, mucus and pus; followed by fever and stomach cramps. ETIOLOGY and TRANSMITION: Shigella species are classified by four serogroups: Serogroup A: S. dysenteriae (12 serotypes) Serogroup B: S. flexneri(6 serotypes) Serogroup C: S. boydii(23 serotypes) Serogroup D: S. sonnei (1 serotype) Humans are the primary reservoir of Shigella species, with captive subhuman primates as accidental hosts. In developing countries with prevailing conditions of inadequate sanitation and overcrowded housing, the infection is transmitted most often by the excreta of infected individuals via direct fecal-oral contamination. Flies may contribute to spread from feces to food. The most common species, S dysenteriae and S flexneri, are also the most virulent. Homosexual men are also at increased risk for direct transmission of Shigella flexneri infections, and chronic, 40 recrudescent illness complicating HIV infection has been reported. Shigella causes dysentery that results in the destruction of the epithelial cells of the intestinal mucosa in the cecum and rectum. Some strains produce enterotoxin and Shiga toxin, similar to the verotoxin of E. coli O157:H7. PATHOGENESIS: Shigella invade epithelial cells of small intestine. The agent proliferates rapidly in the small bowel and attaches to enterocytes, where it replicates within the cytoplasm. Endocytosis is essential for virulence and the virulence factor is encoded by a plasmid. Replicating shigellae kill infected cells and then spread to adjacent cells and into the lamina propria.) Grossly, the rectosigmoidal lesions of shigellosis resemble those of ulcerative colitis. There is proximal extension of erythema, edema, loss of vascular pattern, focal hemorrhage, and adherent layers of purulent exudate. Biopsy specimens from affected areas are typically edematous, with capillary congestion, focal hemorrhage, crypt hyperplasia, goblet cell depletion, mononuclear and polymorphonuclear (PMN) cell infiltration, shedding of epithelial cells and erythrocytes, and microulcerations. A patchy inflammatory pseudomembrane, composed of neutrophils, fibrin and necrotic epithelium, is commonly found on the most severely affected areas. The stool may contain blood, mucus, or pus. The most common symptoms are fever, nausea, vomiting, stomach cramps, and straining to have a bowel movement. In rare cases, young children may have seizures. Symptoms can take as long as a week to show up, but most often begin two to four days after ingestion. Symptoms usually last for several days, but can last for weeks. Stages: 1. Catharal colitis; 2. Fibrinous colitis; 3. Ulcerative colitis 4. Healing of ulcers. Complications: General complications are dehydration, electrolyte imbalance, circulatory failure and renal failure. Local complications consist of intestinal perforation, rectal bleeding, paralytic ileus, intussusceptions and passage of intestinal casts. Pneumoperitoneam, portal pyemia and multiple abscesses in the liver may occur rarely. Sequelae include arthritis (post- dysenteric arthritis), conjunctivitis, iritis and peripheral neuropathy. Amebic dysentery, also known as amebiasis. Amoebiasis is worldwide parasitic disease that is especially prevalent in the tropical countries. E. Histolytica occurs in two stages; a motile trophozoites stage and a non-motile cystic stage. Reservoir is human, an infected person suffering from the disease or an asymptomatic carrier discharging eggs in stools. Sources are water or food contaminated by excreta of patient or carries containing the cysts of E. histolytica. Amoebiasis is a parasitic disease caused by the organism, Entamoeba histolytica. It is the second leading cause of death from parasitic disease in the world. Infection involves ingestion of cysts in focally contaminated food or water. These cysts pass through the 41 gastrointestinal tract. These cysts may then be excreted in stool, completing the life cycle. Intestinal amebiasis can be subdivided into several categories: Asymptomatic infection. Most persons with amebiasis have no noticeable symptoms. Even though these individuals may not feel ill, they are still capable of infecting others by person-to-person contact or by contaminating food or water with cysts that others may ingest, for example, by preparing food with unwashed hands. Chronic non-dysenteric infection. Individuals may experience symptoms over a long period of time during a chronic amebiasis infection and experience recurrent episodes of diarrhea that last from one to four weeks and recur over a period of years. These patients may also suffer from abdominal cramps, fatigue, and weight loss. Amebic dysentery. In severe cases of intestinal amebiasis, the organism invades the lining of the intestine, producing sores (ulcers), bloody diarrhea, severe abdominal cramps, vomiting, chills, and fevers as high as 104-105°F (40-40.6°C). In addition, a case of acute amebic dysentery may cause complications, including inflammation of the appendix (appendicitis), a tear in the intestinal wall (perforation), or a sudden, severe inflammation of the colon (fulminating colitis). Ameboma. An ameboma is a mass of tissue in the bowel that is formed by the amebiasis organism. It can result from either chronic intestinal infection or acute amebic dysentery. Amebomas may produce symptoms that mimic cancer or other intestinal diseases. Perianal ulcers. Intestinal amebiasis may produce skin infections in the area around the patient's anus (perianal). These ulcerated areas have a "punched-out" appearance and are painful to the touch. COMPLICATIONS: complications include the development of intra-abdominal abscesses, liver abscess, brain abscess, amoebic liver abscess rupture through the diaphragm (causing empyema and fistulas), rectovaginal fistulas, perianal disease, and bacterial super-infections. 42 DEFENITIONS: SIRS –Systemic inflammatory response syndrome - the clinical syndrome that results from a deregulated inflammatory response or a noninfectious insult. SIRS may be caused by: • hypoxic-reperfusion damage (ischemia) • infection (endotoxin, other microbial toxins or microorganisms) • primary mediators (histamin, anaphylatoxins /C3a, C5a /) • complexes antigen-antibody (immune-mediated organ injury) • multiple trauma Sepsis is most frequent example of severe SIRS caused by infectious injury. Diagnostic criteria (Presence of SIRS is indicated by the presence of minimum 2 signs). Symptoms Assessed factors Body temperature >38oC or <36oC Pulse rate >90 /min Rate of breathing or Frequency of breathing >20 /min PCO2 (arterial blood) PaCO2 <32 mm Hg White blood count or >12 000/mm3 or <4 000/mm3 I/T ratio (immature (band) forms) >10% Sepsis is defined as at least two of the following signs and symptoms (SIRS) that are both present and new to the patient and suspicion of the new infection. Traditionally, sepsis was viewed as an excessive systemic pro-inflammatory reaction to an infection. More recently, it has been proposed that the early phase of hyper-inflammation is followed or overlapped by a prolonged state of immunosuppression, referred to as sepsis-induced immunoparalysis. This immunoparalytic state is characterized by impaired innate and adaptive immune responses and may play a central role in tissue damage, multiple organ failure, and death induced by sepsis. Presence of infection with bacteremia is not a synonym for sepsis nowadays! Infection - microbial phenomenon, characterized by an inflammatory response to the presence of microorganisms or the invasion of normally sterile host tissue by those organisms. Bacteraemia - presence of a small number of bacteria in the blood which do not multiply significantly (blood is a transporting medium for many bacteria). 43 Sepsis is different from the other infections due to following characteristics: Sepsis may be caused by different microorganisms - polyetiological. Sepsis is not contagious and can‘t be reproduced experimentally There are no definite stages usually observed for other infections (incubation period, prodromal period, height of the disease). The clinical manifestations of sepsis are stereotyped, nonspecific and don‘t depend on the etiology. Immune response against sepsis is absent. So, Sepsis may be defined as a generalized acyclic infectious disease caused by different microorganisms and characterized by extremely transformed reactivity of the organism. Obviously, most infections do not trigger the septic reaction. Two people can have infections in the same tissues, caused by the same microbe, yet one person will develop sepsis and the other person will not. This difference indicates that other factors, beyond the type of tissue and the kind of microbe, are involved in the development of sepsis. Severe sepsis: includes SIRS and at least one of the following signs hypoperfusion or organ dysfunction that is new and not explained by other known etiology of organ dysfunction Clinical manifestations of organ dysfunction due to hypoperfusion for diagnosis of severe sepsis Sepsis-induced hypotension Lactate above upper normal laboratory limits Urine output < 0.5 mL/kg/hr for more than 2 hours despite adequate fluid refill Acute lung injury with PaO2/FIO2 < 250 in the absence of pneumonia as infection source Acute lung injury with PaO2/FIO2 < 200 in the presence of pneumonia as infection source Creatinine > 2.0 mg/dL (176.8 µmol/L) Bilirubin > 2 mg/dL (34.2 µmol/L) Platelet count < 100,000 µL Coagulopathy (international normalized ratio > 1.5) (Adapted from Levy MM, Fink MP, Marshall JC, et al: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256). Septic shock is defined as severe sepsis associated with refractory hypotension (BP<90/60) despite adequate fluid resuscitation and/or a serum lactate level >=4.0 mmol/L. 44 CLASSIFICATION of SEPSIS According to the portal of the infection entry: Surgical; Obstetric-gynecological; Urologic; Ottogenous; Odontogenous and etc. Umbilical Cryptogenic etc The portal of infection entry may or may not be evident. If evident, we can observe nearby lymphangitis, lymphadenitis; the lymphatics and lymph nodes that drain the inflamed tissue show reactive inflammatory changes in the form of lymphangitis and lymphadenitis. This response represents either a nonspecific reaction to mediators, released from inflamed tissue or an immunologic response to a foreign antigen. The affected lymph nodes may show hyperplasia of lymphoid follicles (follicular hyperplasia) and proliferation of mononuclear phagocytic cells in the sinuses of lymph node (sinus histiocytosis). According to etiology: almost any bacterial, fungal or protosoal infection may be the causative agent for sepsis 1.Bacteria: Gram-positive (Staphylococcal, Streptococcal); Gram-negative (Klebsiella pneumoniae, Salmonella, Haemophilus influenza, Escherichia coli, proteus); 45 Anaerobic bacteria 2. Fungi 3. Protozoa Risk Factors • Extreme Age (under 1 and > 65 years) • Surgical/Invasive procedures in anamnesis • Malnutrition • Alcoholism • Usage of broad-spectrum antibiotics • Chronic illness • Chemo-, radiotherapy, therapy with steroids. • Immune deficiency disorders • Antibiotic resistance of etiological factor Clinico-morphological forms of sepsis Septicopyemia is the dissemination of small septic thrombi in blood, which cause their effects at the site where they are lodged. This can result in the pyaemic abscesses or septic infarcts. a) Pyemic abscesses are multiple small abscesses in various organs such as in the cerebral cortex, myocardium, lungs and renal cortex, resulting from very small emboli fragmented from septic thrombus. Microscopy of pyemic abscess shows the central zone of necrosis containing numerous bacteria, surrounded by a zone of suppuration and an outer zone of acute inflammatory cells. b) Septic infarcts are the result of lodgment of larger fragments of septic thrombi in the arteries with formation of relatively larger foci of necrosis, suppuration and acute inflammation e.g. septic infarcts of the lungs, liver, brain, and kidneys from septic thrombi of leg vein or in case of acute bacterial endocarditis. Septicaemia – presence of rapidly multiplying, highly pathogenic bacteria in tissues without secondary abscesses. The main pathogenic effects are due to toxemia, release of cytokines. 46 Pic. 2 The interrelationship between SIRS, Sepsis and Infection (adapted from Chest 1992; 101: 1644-55)5 Pathophysiology of Sepsis and Septic Shock Sepsis begins as a typical inflammatory response to an infection. Like any inflammation, it starts with the local mobilization of macrophages and neutrophils and the activation of the complement and coagulation systems. When working properly, the innate immune mechanisms are rapidly mobilized in the region of a new infection. At the height of the response, invading microbes are overwhelmed, deactivated, and destroyed. Next, local debris is removed; the pro-inflammatory molecules, the activated complement, and the activated clotting factors are neutralized; and the production of new pro-inflammatory molecules stops. In a typical inflammatory reaction, the local pro-inflammatory processes are balanced by systemic anti-inflammatory processes and are automatically terminated within a short time. In sepsis, however, cytokine production continues unending and the circulatory spread of the cytokines then causes increased cytokine production at distant sites. In sepsis, pro-inflammatory molecules can be found in high concentrations throughout the circulation. Sepsis has been shown to develop when the innate immune response becomes amplified and deregulated, leading to an imbalance between pro-inflammatory and anti-inflammatory responses, very rapidly resulting in uncontrolled hypotension, hypoperfusion i.e. septic shock with multi- organ dysfunction. 47 The role of causative agent for initiation of the process. Originally sepsis was described, and strongly considered to be, a disease specifically related to Gramnegative bacteria. This is because sepsis was thought to be a response to endotoxin. In fact, some of the original studies of sepsis showed that Gram-negative bacteria were among the most common causes of sepsis. This resulted in a number of trials that focused on Gram-negative therapies, and even highly specific therapies for endotoxin, which were felt to be potentially useful treatments for sepsis. Pic. 3. Inflammatory Responses to Sepsis. (source - The new England journal of medicine 355;16, 2006) Gram-positive and gram-negative bacteria and fungi have unique cell-wall molecules called pathogen-associated molecular patterns that bind to patternrecognition receptors (toll-like receptors [TLRs]) on the surface of immune cells. The lipopolysaccharide of gram-negative bacilli binds to lipopolysaccharidebinding protein, CD14 complex. The peptidoglycan of gram-positive bacteria and the lipopolysaccharide of gram-negative bacteria bind to TLR-2 and TLR-4, respectively. Binding of TLR-2 and TLR-4 activates intracellular signaltransduction pathways that lead to the activation of cytosolic nuclear factor κB (NF-κB). Activated NF-κB moves from the cytoplasm to the nucleus, binds to transcription initiation sites, and increases the transcription of proinflammatory and antiinflammatory cytokines (TNF-α, interleukin-1β, and interleukin-10). Cytokines regulate a variety of inflammatory responses, including the migration of immune cells to the infection, which is a crucial step in containing a localized infection and preventing it from becoming systemic. However, a dysregulated cytokine release may lead to vasodilation and increased capillary permeability. The resulting 48 leakage syndrome can cause hypotension, hemoconcentration, macromolecular extravasation, and edema, which are frequent findings in septic patients. TNF-α and interleukin-1β are proinflammatory cytokines that activate the adaptive immune response but also cause both direct and indirect host injury. Interleukin-10 is an antiinflammatory cytokine that inactivates macrophages and has other antiinflammatory effects. The mechanism of the onset of gram-positive and gram-negative sepsis is a bit different: Endotoxin is a lipopolysaccharide in the cell wall of Gram-negative bacteria. When it gets into the circulation, endotoxin strongly activates the coagulation and complement systems throughout the body. Also it causes directly paralysis of blood vessels, causing uncontrolled hypotension and hypoperfusion. Exotoxins are another class of sepsis-worsening molecules that are produced by Gram-positive bacteria. Exotoxins are superantigens, meaning that they bypass the standard immune activation process and directly trigger host cells to release cytokines. Disturbances in vascular tone regulation are based on local microcirculatory changes. Releasing of NO seems to be the main reason for dysfunction of vascular smooth muscle in sepsis. NO causes a hyperpolarization of smooth muscle plasma membranes, rendering them irresponsible to catecholamines and causing an uncontrolled vasodilation. Massive release of cytokine TNF-α in response to severe tissue injury or infection results in profuse systemic vasodilatation, increased vascular permeability and intravascular volume loss. Vascular alterations in septic shock are mainly due to the effects of mediators on vascular smooth muscle. In addition, these patients may have relative vasopressin or cortisol deficiencies, leading to further deterioration of refractory shock. Endothelial changes: key functions of the endothelium are selective permeability, vasoregulation, and provision of an anticoagulant surface. Proteases, oxidants, prostaglandins, and leukotrienes injure endothelial cells cause increasing of permeability, further vasodilation and alteration of the procoagulant–anticoagulant balance. Endothelial dysfunction leads to an inability of the endothelial cells to maintain vascular tone with loss of blood pressure. In addition, endothelial damage leads to capillary leak with intravascular volume depletion and edema formation in involved organs. Cytokines also activate the coagulation cascade. This leads to the deposition of fibrin by the coagulation cascade in a sticky meshwork that helps to fence in and restrict the spread of microbes from the vicinity. Systemic activation of 49 coagulation pathway may occur leading to microthrombi throughout the body and result in disseminated intravascular coagulation (DIC), bleeding and death. Futher activation of endothelial cells, platelets, neutrophils, plasmatic hemocoagulation system and complement results in chemotaxis of neutrophils, interstitial edema and microthrombi formation; edema additionally compresses lymphatic and blood stream, causing hypoxia and organ dysfunction as consequence causing irreversible tissue damage - usually the end result of different coexisting mechanisms and Multiple Organ Dysfunction Syndrome formation due to necrosis caused by ischemia, direct lysis of the cells, or apoptosis induced by TNFa. Pic.4. Procoagulant Response in Sepsis. (source - The new England journal o f medicine 355;16, 2006) Finally, as a result of the overactivation of the cytokine and cellular defense systems in the progressive and final stages of septic shock, susceptibility to bacterial infections increases, resulting in joining of secondary infections. Morphology of MODS in sepsis. Lungs are usually an early casualty in sepsis, regardless of the location of the initial infection. The surface area of the vascular endothelium of the lung is large, and when a septic reaction begins haphazardly disrupting endothelial areas in the body, the lung is likely to suffer significant damage. Regions of the lung with damaged endothelia become filled with neutrophils and macrophages. Interstitial spaces develop edema, fibrin is deposited, and surfactant is reduced. These regions of the lung become heavy and poorly compliant and local gas exchange is minimal. 50 To make matters worse, the phenomenon of hypoxic pulmonary vasoconstriction (HPV) is counteracted in sepsis. HPV is a protective mechanism that normally redirects arterial blood away from any nonfunctioning parts of the lung to better ventilated areas. In sepsis, however, circulating inflammatory molecules reduce the ability of lung arterioles to constrict. Without HPV, blood will continue to flow through useless regions of the lung, and the body's growing systemic hypoxemia worsens. Increasing lung dysfunction eventually leads to lung failure. In sepsis, lung failure takes the form of acute respiratory distress syndrome, or ARDS. Acute respiratory distress syndrome is a sudden-onset pulmonary edema caused by endothelial injury in the lungs. During ARDS, leaky pulmonary capillaries allow alveoli to be flooded, and the lungs get heavy and are poorly compliant. Chest films of ARDS patients show diffuse or patchy infiltrates bilaterally. Gas exchange is reduced, and the patient becomes dyspneic and hypoxemic. One characteristic of the hypoxemia in ARDS is a low arterial oxygen level that remains low at all levels of oxygen supplementation. In other words, the ratio of the concentration of the arterial O2 to the concentration of the inspired O2 remains below 200: PaO2/FIO2 <200. Sepsis is the most frequent cause of ARDS, and ARDS develops in approximately half of all patients with severe sepsis or septic shock. On average, ARDS has a mortality rate of 30% to 40%, but in sepsis, ARDS has a mortality rate >50%. The most frequent etiology is pneumonia, followed by nonpulmonary infections. Heart. As the sepsis continues, the heart muscle begins to weaken due to the depressant effect of some of the circulating inflammatory molecules; however, the weakened ventricles also stretch, so the dilated ventricles pump extra blood with each stroke. The additional stroke volume partly compensates for the heart's decreased pumping power. In this way, the cardiac output (blood volume pumped per minute) can remain fairly constant or even increase during a bout of sepsis. Kidneys. Like the lung, the kidney's function is entirely dependent on maintaining a significant area of intact vascular endothelium. When the septic reaction invades the kidneys, neutrophils and macrophages begin to fill the interstitial tissue and the endothelial cells of the blood vessels are activated and damaged. At the same time, the kidneys—indeed all body tissues—become underperfused and hypoxic. At first, kidney dysfunction appears as a reduced glomerular filtration rate and an increase in serum creatinine levels. If the sepsis continues, acute tubular necrosis develops, which can eventually lead to acute renal failure. Gastrointestinal Tract. The spreading hypoperfusion of sepsis limits the oxygen supply to the intestines. As aerobic metabolism is superseded by anaerobic metabolism, lactate levels build in the portal vessels, and the pH drops inside the gut. Hypoxia and acidosis stress the epithelium that lines the gastrointestinal tract, 51 and its natural barrier functions (including protection against gut microbes) are weakened. Bacteria and toxic molecules from the gut lumen slip through the gut wall and into the bloodstream and the lymphatics. Sepsis typically causes small painless erosions in the mucosa (especially in the upper GI tract), resulting in a continual seepage of blood. In severe sepsis or septic shock, the hypoperfusion can also immobilize the intestines, which then develop paralytic ileus. Liver. During sepsis the liver plays a key role, acting as a first line of defense in the clearance of the infectious agents and their products; but sepsis can induce liver damage through hemodynamic alterations or through direct and indirect assault on the hepatocytes. The sepsis-induced liver dysfunction leads to a spillover of bacteria, bacterial toxins, and debris into the circulation. Elevated liver enzymes and coagulation defects may occur. A decreased ability to excrete toxins such as ammonia can lead to encephalopathy. Nervous System. Sepsis often causes acute brain dysfunction, characterized by fluctuating mental status changes, inattention, and disorganized thinking. The effects on the brain are caused by both inflammatory and non-inflammatory processes, which may induce significant alterations in vulnerable areas of the brain. The problems begin when circulating inflammatory molecules disrupt the endothelium of the blood vessels along the blood-brain barrier (BBB). The leaky BBB lets inflammatory molecules, along with infiltrating white cells, into the neural tissue. Subsequently, edema and collections of cells around arterioles hinder the entry of oxygen and nutrients and the exit of metabolic wastes. In this situation, neurons shut down and cerebral functions slow. Pict. 5. Pathophysiology of severe sepsis and septic shock. (Adapted from Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med 2009;37(1):293) PAMPS – pathogen associations molecular patterns, DAMPS - damage associations molecular patterns. 52 Outcome of Sepsis The mortality of septic shock ranges from 25 to 75% according to age (the older the patient, the poorer the outcome), the underlying cause, and patient management. In clinical practice the outcome is often determined by the degree of acute cardiac muscle damage (on the top of any pre-existing heart disease) and the capacity of the lung alveolar lining cells to regenerate whilst the fibrosis is inhibited. It must be remembered that modern intensive care itself, with positive-pressure ventilation, high inhaled oxygen concentrations and powerful toxic drugs including inotropes, can incur and may reinforce the pathology of shock: the balance between undertreatment and overtreatment is often difficult to negotiate. INFECTIVE (BACTERIAL) ENDOCARDITIS (IE or BE) (Бактериальный/Септический/Инфекционный эндокардит) Bacterial endocarditis is serious infection of the valvular and mural endocardium caused by different forms of microorganisms and is characterized by typical infected and friable vegetations. To say it briefly, it is a form of sepsis with predominate affection of the valvular casps. Etiology and Pathogenesis are connected to bacteraemia because of presence of any possible source of infection in the organism (pneumonia, infection of the urinary, gastrointestinal and biliary tracts, dental inflammatory diseases, urethral catheterization, minor invasive procedures as cystoscopy and rectoromanoscopy). Intravenous drug misuse and drug-abuse is an increasingly important cause of tricuspid and pulmonary valve infection. Up to 10% of patients with endocarditis have no obvious portal of entry for the organism. Bacteria causing BE on entering the bloodstream from any of the above-mentioned sources are implanted on the cardiac valves or mural endocardium because they have surface adhesion molecules which mediate their adherence to injured endocardium. There are several predisposing conditions which explain the development of bacterial implants on the valves: 1. The circulating bacteria are lodged much more frequently on previously damaged valves, than on healthy valves (rheumatic heart disease, congenital heart defects and prosthetic valves). The disease is called secondary bacterial endocarditis. If BE develops on intact valves it is called primary bacterial endocarditis or Chernogubov‘s disease. 2. Conditions producing haemodynamic stress on the valves are liable to cause damage of endothelium, favoring the formation of platelet-fibrin thrombi which get infected from circulating bacteria. 53 BE is subdivided into 2 clinical forms: 1. Acute bacterial endocarditis (ABE) is fulminant and destructive acute infection of the endocardium caused by highly virulent bacteria, usually resulting in death of the patient in 2-6 weeks. Staphylococcus aureus, Streptococcus pyogenes and Enterococci are the most common etiological agents. The mitral and aortic valves are usually involved, but the tricuspid valve is often infected in intravenous drug-abused persons. Blood culture is nearly always positive. Rapidly changing cardiac murmurs occur as valve cusps are being destroyed. Septicaemia and embolism occur very commonly, destruction of cusps is the reason for acute heart failure. Mortality is over 50%, even with intensive treatment. Early surgery may be beneficial in some patients. Morphology: Compared to subacute endocarditis, the vegetations are larger and loosely attached to cusps. They consist mainly of fibrin, necrotic masses containing large clusters of organisms surrounded by neutrophil polymorphs. Rapid and severe cusp destruction is common. Involvement and rupture of chordae tendineae is common. Local invasion of bacteria to underlying myocardium or adjacent aorta causes abscess formation. 2. Subacute bacterial endocarditis (SABE) or endocarditis lenta (lenta = slow) Subacute infective endocarditis is usually caused by bacteria of low virulence, most commonly the ‗viridans‘ group of a-hemolytic Streptococci which is a part of the normal flora of the mouth, pharynx and is important in periodontal infection. Group D Streptococci (gut commensals) and Staphylococcus epidermidis (skin commensal), which infect venous catheters and pacemaker wires, are also important. Other Gram-negative bacilli, Coxiella, mycoplasma and fungi are infrequent. Endocarditis due to fungi tends to occur in intravenous drug-abusers; in immunosuppressed patients, infection Candida and Aspergillus are most common as causative agents. Morphology Subacute infective endocarditis starts insidiously with fever, malaise and mild anaemia. Petechiae may occur in the skin (Janeway lesions), mucous membranes and retina (Roth‘s spots) and ‗splinter‘ haemorrhages are seen under the nails. Some of these changes are probably embolic in origin. The spleen is usually enlarged and palpable. Cardiac murmurs may be the result of the previous heart disease or may develop and change as the vegetations progress. As vegetations grow and disintegrate, emboli may be formed. Haematuria may result from either renal infarction or glomerulonephritis, the latter being mediated by immune complex reactions of hypersensitivity. Osler's nodes are painful, red, raised lesions found on the hands and feet are another example of lesions due to type III reactions 54 of hypersensitivity. Untreated subacute infective endocarditis is invariably fatal within a few months from heart failure, embolic phenomena or renal failure. Blood cultures and ultrasoundgraphy of heart to visualize the valves are mandatory investigations. Appropriate antimicrobial therapy reduces the mortality, which still remains high even when the infecting organism is identified. Microscopically, the vegetations of BE consist of 3 zones 1) The outer layer or cap consists of eosinophilic material composed of fibrin and platelets. 2) Underneath this layer is the basophilic zone containing colonies of bacteria. However, bacterial component of the vegetations may be lacking in treated cases. 3) The deeper zone consists of a non-specific inflammatory reaction in the cusp itself. COMPLICATIONS A. Cardiac complications. These include the following: * Valvular stenosis or insufficiency * Perforation, rupture, and aneurysm of valve leaflets * Abscesses in the valve ring * Myocardial abscesses * Suppurative pericarditis * Cardiac failure as the result of one or more complications listed above. B. Extracardiac complications. Since the vegetations in BE are typically friable (especially in ABE), they tend to get dislodged and give rise to embolism which is responsible for very common and serious extra-cardiac complications. These are as under: * Formation of thrombi in the left side of the heart with systemic thromboembolism (infarcts of spleen, kidneys, and brain), abscesses and aneurysms. * Formation of thrombi in the right side of the heart with pulmonary thromboembolism and pulmonary abscesses. * Petechiae may be seen in the skin and conjunctiva due to either emboli or toxic damage to the capillaries. * In SABE, there are painful, tender nodules on the fingertips of hands and feet called Osler’s nodes, while in ABE there is appearance of painless, nontender subcutaneous maculopapular lesions on the pulp of the fingers called Janeway’s spots. In either case, their origin is due to the toxic or allergic inflammation of the vessel wall. * Focal necrotising glomerulonephritis is seen more commonly in SABE than in ABE. Occasionally diffuse glomerulonephritis may occur. Both these have 55 their pathogenesis in circulating immune complexes (hypersensitivity phenomenon). Treatment of BE with antibiotics in adequate dosage kills the bacteria but complications and consequences of healed endocardial lesions may occur even after successful therapy. The causes of death are cardiac failure, persistent infection, and embolism to vital organs, renal failure and rupture of mycotic aneurysm of cerebral arteries. SPECIFIC TYPES OF INFECTIVE ENDOCARDITIS Besides BE, various other microbes may occasionally produce infective endocarditis, which are named according to the etiologic agent causing it. These include the following: 1. Tuberculous endocarditis. Though tubercle bacilli are bacteria, tuberculous endocarditis is described separately from the bacterial endocarditis due to specific granulomatous inflammation found in tuberculosis. It is characterized by presence of typical tubercles on the valvular as well as mural endocardium and may form thromboemboli. 2. Syphilitic endocarditis - involvement of aortic valve due to syphilitic aortitis, characterized by necrosis of the aortic media, gradual weakening and stretching of the aortic wall, and aortic aneurysm of ascending aorta. Damage and scarring of the ascending aorta commonly leads to dilation of the aortic ring, separation of the valve cusps and regurgitation of blood through the aortic valve (aortic insufficiency). 3. Fungal endocarditis. (Candida albicans, Histoplasma capsulatum, Aspergillus, Mucor, coccidioidomycosis, cryptococcosis, blastomycosis and actinomycosis). Opportunistic fungal infections like candidiasis and aspergillosis are seen most commonly in patients under long-term antibiotic therapy, intravenous drug abusers and HIV-infected. Fungal endocarditis produces appearance similar to that in BE but the vegetations are bulkier. 4. Viral endocarditis. There is only experimental evidence of existence of this entity. 5. Rickettsial endocarditis. Very rare. Lesions Simulating Infective Endocarditis Non-bacterial Thrombotic Endocarditis Non-bacterial thrombotic endocarditis (marantic or terminal endocarditis) consists of small thrombi on the heart valves, usually in a patchy fashion along the lines of cusp closure of the mitral and aortic valves. They are probably caused by a hypercoagulable state since identical lesions occur in any patient with acute DIC. They are usually asymptomatic but both systemic embolism and secondary 56 infection of the thrombi are recognized complications. Characteristically, this condition occurs in patients with a debilitating illness such as cancer or tuberculosis. Libman–Sacks Endocarditis This may develop in patients with systemic lupus erythematosus and can involve the mitral, aortic, or tricuspid valves. The vegetations are sterile, platelet-rich and rarely exceed 2 mm in size. Fibrinoid necrosis is a characteristic feature. The group includes: 1. Influenza viral infection 2. Parainfluenza viral infection 3. Adenoviral infection 4. Respiratory- syncytial viral infection 5. Rhinoviral infection INFLUENZA (Грипп) Influenza is an acute, usually self-limited, infection of the upper and lower airways, caused by influenza virus. Its general clinical features range from a mild afebrile illness similar to common cold by appearance of sudden fever, headache, myalgia, malaise, chills and respiratory tract manifestations such as cough, sore throat to a more severe form of acute respiratory illness and lymphadenopathy. ETIOLOGIC AGENT. Influenza virus is a single-stranded RNA virus belonging to coronaviruses. There are 3 distinct types of virus: A, B and C. Influenza type A is responsible for most serious and severe forms of outbreaks in human beings while types B and C cause a milder form of illness. Type A influenza virus is subtyped based on its 2 viral surface antigens hemagglutinin (H) and neuraminidase (N). There are 16 distinct H subtypes of type A influenza viruses. N antigen of influenza A exists in 9 subtypes. The main effects of the virus on the organism are suppression of phagocytes migration and severe increasing of vascular permeability. 57 Major antigenic variation in H or N antigens is called antigenic shift while minor variation is termed antigenic drift. In general, population at high risk are immunosuppressed patients, elderly individuals and infants. EPIDEMIOLOGY: Influenza is highly contagious, and epidemics often spread around the world. New strains emerge regularly, often from animal hosts; infect humans in parts of the world where humans and animals live in close contact; and then disseminate rapidly. The avian influenza virus (―bird flu‖) strain that emerged in 2003 and still continues to spread around the globe is designated A (H5N1). In 2009, a novel influenza A virus, designated H1N1 (―swine flu‖), emerged in Veracruz, Mexico, and swiftly spread globally as a pandemic infection. The potential seriousness and rapidity of the H1N1 strain is evidenced by the occurrence of approximately 10,000 deaths in the United States alone within 7 months of its first being identified. This strain of influenza A virus has produced significant mortality in infected children and pregnant women. Because epidemic influenza virus antigens change so often, host immunity that develops during one epidemic rarely protects against the next one. TRANSMITTION: Influenza spreads from person to person by virus-containing respiratory droplets and secretions. When it reaches the respiratory epithelial cell surface, the virus binds and enters the cell by fusion with the cell membrane, a process mediated by a viral glycoprotein (hemagglutinin) that binds to sialic acid residues on human respiratory epithelium. Once inside, the virus directs the cell to produce progeny of viruses and causes cell death. Infection usually involves both the upper and lower airways. Destruction of ciliated epithelium, disturbances of mucociliary clearance, predisposing to bacterial pneumonia, especially with Staphylococcus aureus and Streptococcus pneumoniae. PATHOLOGY: Mild and moderate forms of Influenza are characterized by necrosis and desquamation of ciliated upper and middle respiratory tract epithelium, associated with a predominantly lymphocytic inflammatory infiltrate. The severe forms are characterized by involving of the lungs and formation of viral pneumonia. Morphologically lungs are called ―large patchy lung‖ due to altering foci of red (areas of hemorrhagic inflammation) and grey foci (compensatory emphysema). This form of infection can leads to some specific complications, like hemorrhagic meningitidis. In high percentage of cases severe forms of infection have a lethal outcome. 58 Bird Flu ((Influenza A/H5N1) H5N1 subtype of the influenza type A, also called SARS-associated coronaviruses (SARS-CoV), virus infection causes severe acute respiratory syndrome (SARS) which is the human form of bird flu or avian influenza with having similar symptomatology. Humans do not have immune protection against avian viruses. SARS is a potentially fatal viral respiratory illness with the incubation period of 2 to 7 days, sometimes ranging up to 10 days. CLINICO-PATHOLOGICAL FEATURES. Typically, the disease begins with influenza-like features such as fever, cough, dyspnoea, sore throat, muscle aches and eye inflammation. Late, manifestations of acute respiratory distress syndrome due to viral pneumonia and acute renal failure develop. In the epithelium of the upper respiratory tract multinucleated syncytial cells without viral inclusions have also been observed. LABORATORY DIAGNOSIS. The following abnormalities in laboratory tests are noted: 1. Lymphopaenia, mostly due to a decrease of CD4+ T cells. 2. Thrombocytopaenia. 3. Elevation of liver ferments: aminotransferases, creatine kinase and LDH. 4. Virus detection by means of reverse transcriptase-PCR from respiratory samples, plasma, urine or stool. 5. Detection of serum antibodies by ELISA or immunofluorescence. Swine Flu (Influenza A/H1N1) CLINICAL FEATURES. The disease has the usual flu-like clinical features, but additionally one-third of cases have been found to have diarrhoea and vomiting. Human beings do not have immune protection by antibody response against H1N1 influenza type A and the usual seasonal flu vaccine does not provide protection against H1N1, personal hygiene and prophylaxis remain the mainstay of further spread of the disease. PARAINFLUENZA (Парагрипп) ETIOLOGY: Parainfluenza viruses cause acute upper and lower respiratory tract infections (laryngotracheobronchitis), particularly in young children. They are enveloped, single-stranded RNA viruses. EPIDEMIOLOGY: This condition is common in children under the age of 3 years. The infection is highly contagious, and the disease is present worldwide. These viruses spread from person to person through infectious respiratory aerosols and secretions. PATHOLOGY: Parainfluenza viruses infect and destroy ciliated respiratory epithelial cells and elicit an inflammatory response. In very young children, this 59 process frequently extends into the lower respiratory tract, causing bronchiolitis and pneumonitis. In young children, where the trachea is narrow and the larynx is small, the local edema in laryngotracheitis compresses the upper airway enough to obstruct breathing and cause false croup. Clinically, parainfluenza infection is associated with fever, hoarseness and cough. A barking cough as well as inspiratory stridor is common. In older children and adults, symptoms are usually mild. ADENOVIRAL INFECTION (Аденовирусная инфекция) Adenoviruses cause acute, self-limiting, febrile infections, with inflammation of the respiratory or ocular mucous membranes or both. ETIOLOGY and TRANSMITTION: Adenoviruses are DNA viruses classified according to 3 major capsid antigens (hexon, penton, and fiber). Transmission occurs by direct inoculation into the eye by fecal-oral contamination (adenoviruses may persist in the GI tract for years after infection) or by inhalation of an infected droplet. The incubation period usually is less than 1 week. Although the acute illness lasts less than 5 days, it may be followed by a prolonged asymptomatic reinfection. In immunocompetent hosts, most adenovirus infections are asymptomatic; when infections are symptomatic, a broad spectrum of clinical manifestations is possible depending on the type of virus responsible for the onset of the disease. MORPHOLOGY: inflammation of upper respiratory airways (rhinitis, pharyngitis, laryngotracheobronchitis). Rare adenoviral syndromes in infants include severe bronchiolitis and pneumonia. In some persons infection with adenovirus may also cause false croup. A separate syndrome involves conjunctivitis, pharyngitis, and fever (pharyngoconjunctival fever). Fever is usually present. Lymphodenopathy in regional lymph nodes and moderate hepatosplenomegaly is common. Some types of Adenovirus may be responsible for cystitis and GIT involvement. COMPLICATIONS: Common but serious complications include pneumonia (viral or secondary bacterial), middle ear infection or otitis media and brain infections or meningitis. 60 PLAGUE (Чума) Plague is a highly contagious, often fatal infection that is usually accompanied by enlarged, painful regional lymph nodes (buboes). Historically, plague caused massive epidemics that killed much of the civilized world. ETIOLOGY: Plague is caused by Yersinia (Pasteurella) pestis. Yersinia pestis is a short gram-negative rod that stains more intensively at the ends (i.e., bipolar staining), particularly with Giemsa stains. Virulence of the organism Y. pestis is attributed to the elaboration of plague toxins: pesticin and lipopolysaccharide endotoxin. EPIDEMIOLOGY: Y. pestis infection is an endemic zoonosis in many parts of the world, including America, Africa and Asia. The organisms are found in wild rodents, such as rats, squirrels and prairie dogs. Fleas transmit it from animal to animal, and the majority cases in people are the result of infected fleas bites. When infected humans develop plague pneumonia (primary plague pneumonia) and start to discharge many causative agents with transmission from person to person, it is the onset of epidemic. Major plague epidemics have occurred when Y. pestis was introduced into large urban area with a lot of rats, in crowded, squalid cities. The infection spread firstly among rats; then, as they died, infected fleas fed on the human population, causing widespread of the disease. The Black Death pandemic in mid–14th-century (1347– 1350) in Europe killed about a third of Europe‘s population, perhaps 34 million people. In the United States, 30 to 40 cases of plague occur annually, mostly in the Southwest desert. PATHOGENESIS: After inoculation into the skin, Y. pestis is phagocytozed by neutrophils and macrophages. Microorganisms, ingested by neutrophils, are killed, but those engulfed by macrophages survive and replicate intracellularly. The bacteria are carried to regional lymph nodes, where they continue to multiply, producing extensive hemorrhagic necrosis. From regional lymph nodes, they disseminate via the bloodstream and lymphatics to the lungs as well. In the lungs Y. pestis produces necrotizing pneumonitis (“secondary plaque pneumonia”) that releases organisms into the alveoli and airways. These are expelled by coughing, enabling pneumonic spread of the disease. 61 CLINICAL FEATURES: There are 4 variants of Y. pestis infection, although they often overlap. All types of plague have a high mortality rate (50% to 75%) if untreated. ■ Bubonic plague begins within 2 to 8 days of the flea bite, with headache, fever and myalgias and with painful enlargement of regional lymph nodes, mostly in the groin, because flea usually bites lower extremities. Affected lymph nodes, known as ―buboes,‖ are frequently enlarged and fluctuant due to extensive hemorrhagic necrosis. Infected patients often develop necrotic, hemorrhagic skin lesions over the buboes, they are black in colour due to hemolysis, hence the name ―black death‖ for this disease. Disease progresses to septic shock within hours to days. Microscopically, the features are as follows: -acute lymphadenitis of the affected nodes -necrosis in the affected nodes and around them. -characteristic mononuclear inflammatory response. -masses of proliferating bacilli in sinusoids of lymph nodes. Typhoidal plague. The main manifestations are due to GIT involvement, including diarrhoea and painful abdomen. The lesions in typhoidal plague are the following: -necrotic foci in visceral lymphoid tissue. -necrotic areas in parenchymal visceral organs. ■ Pneumonic plague results from inhalation of air-borne particles from remnants of animals or usually from infected persons (“primary plaque pneumonia”) or the result of bacilli dissemination in patients with another form of the disease (“secondary plaque pneumonia”). The main clinical manifestations - high fever, cough and dyspnea- begin suddenly. The sputum contains a lot of bacilli. Respiratory insufficiency and endotoxic shock kill the patient within 1 to 2 days. Microscopic features: -necrosis of alveolar walls. -hemorrhagic exudate. -numerous bacilli in the alveolar lumina. -characteristic mononuclear inflammatory response with very scanty neutrophils. ■ Septicemic plague (10% of cases) occurs when bacteria are inoculated directly into the blood and do not produce buboes. Patients die because of septic shock. Fever, prostration occur suddenly resulting in death within 48 hours. All blood vessels contain bacilli. Fibrin casts surround the organisms in renal glomeruli and dermal vessels. 62 ANTHRAX (Сибирская язва) Anthrax is a necrotizing infection caused by Bacillus anthracis, which is a large spore-forming, gram-positive rod. EPIDEMIOLOGY: Anthrax has been known for centuries, and descriptions of the disease were reported in early Hebrew, Roman and Greek records. The major reservoirs are goats, sheep, cattle, horses, pigs and dogs; they release bacilli through feces. Spores in the soil and dead animals are resistant against heat, desiccation and chemical disinfection for years. Humans are infected when spores enter the body through breaks in the skin, by inhalation or by ingestion. Humans may be infected after contact with contaminated animal byproducts, such as hides, wool, and brushes. Anthrax has been a persistent problem in Iran, Turkey, Pakistan and Sudan. One of the largest recorded naturally occurring outbreaks of anthrax occurred in Zimbabwe, when the estimated 10,000 people became infected in 1978 to 1980. In North America, human infection is extremely rare (one case per year for the past few years) and usually results from exposure to imported animal products. However, increased vigilance for anthrax has emerged following a recent bioterrorism episode involving transport of organisms by the postal system. ETIOLOGY and PATHOGENESIS. Bacillus anthracis, is a gram-positive, aerobic bacillus. It is a spore-forming bacillus and the spores so formed outside the body are quite resistant. The spores of B. anthracis germinate in the human body to yield vegetative bacteria that multiply and release a potent necrotizing toxin. In 80% of cases of cutaneous form of anthrax infection remains localized and host immune response eventually eliminates the organism. If the infection disseminates, as occurs when the organisms are inhaled or ingested, the case is usually fatal. Depending upon the portal of entry, three types of human anthrax are known: Cutaneous form occurs after direct contact with the skin. It is the most common form, that accounts for 95% of all anthrax. Necrotic lesion due to vascular thrombosis, haemorrhage and acellular necrosis progresses to Malignant pustule. The patient has an elevated skin papule that enlarges and erodes into an ulcer. Bloody purulent exudate accumulates and gradually darkens to purple or black (―anthrax‖ means ―coal‖) . The ulcer is often surrounded by a zone of brawny edema, which is disproportionately large for the size of the ulcer. Regional lymphadenitis is a predictor of the poor prognosis, since lymphatic invasion precedes septicemia. If infection does not disseminate, cutaneous lesions heal without sequel. Pulmonary form occurs after inhalation of spores of B. anthracis and is also called the ―wool sorters‘ disease‖. The form is mostly fatal. It begins as a flu-like illness that rapidly progresses to respiratory failure and shock. Morphologically 63 malignant pustules in the bronchi are the hallmarks. This is followed by development of primary extensive necrotizing pneumonia and haemorrhagic mediastinitis. Death often ensues within 24 to 48 hours. Gastrointestinal form is rare and is acquired by eating contaminated meat. Stomach or bowel ulceration with regional lymphatics involvement is common. Septicemic anthrax more commonly follows pulmonary anthrax. Disseminated intravascular coagulation is the main complication. Moreover, the bacterial toxin depresses the respiratory center. (Синдром приобретенного иммунодефицита – СПИД) AIDS is a disease that is characterized by profound immunosuppression which leads to opportunistic infections, secondary neoplasms and neurological disorders. In 1981, clinicians in the USA noted unusual frequencies of previously rare conditions – Pneumocystis jirovecii pneumonia and Kaposi‘s sarcoma in previously healthy men. These subjects were homosexual, and were suffering these fatal diseases as a consequence of depleted T-helper cells (CD4-phenotype). The disease was termed the ‗acquired immunodeficiency syndrome‘, or AIDS. By 1984, the etiology of this immunosuppression had been determined as a retrovirus, the modes of viral transmission described, and the geographical spread of the infection was being investigated. ETIOLOGIC AGENT. AIDS is caused by an RNA retrovirus called human immunodeficiency virus (HIV), of which type 1 (HIV-1) is globally distributed, and the second much less frequent type (HIV-2) is mainly restricted to West African countries or people from there. HIV have tropism for CD4 molecules presented on the subpopulation of T-cells, which are the particular targets for HIV. HIV-I virion or virus particle is spherical in shape and 100-140 nm in size. It contains the core having core proteins, chiefly p24 and p18, two strands of genomic RNA and the enzyme - reverse transcriptase. The core is covered by a double layer of lipid membrane derived from the outer membrane of the infected host cell during budding process of virus. The membrane is studded with 2 envelope glycoproteins - gp120 and gp41. 64 There are three important genes coding the components of the virion: i) gag (group antigen) for core proteins, ii) pol (polymerase) for reverse transcriptase, and iii) env (envelope) for the envelope proteins. These genes and viral components act as markers for the laboratory diagnosis of HIV infection. Besides, there is tat (transcription activator) gene for viral functions such as amplification of viral genes, viral budding and replication. HIV/AIDS epidemiology By the end of 2002, there were an estimated 42 million adults and children living with HIV or AIDS. Among them 28.5 million (68%) were living in sub-Saharan Africa, and 6 million (14%) in South and South-East Asia. In 2002, an estimated 5 million adults and children became infected with HIV, and an estimated 3.1 million adults and children died from HIV/AIDS. 2.4 million (77%) of these deaths occurred in sub-Saharan Africa. Sub-Saharan Africa is the region with the highest overall HIV seroprevalence rate in the general adult (15–49 years) population (9% as of end 2002). Of 25 countries with an adult HIV seroprevalence rate above 5% in 2001, 24 are in sub-Saharan Africa. The only other country with an adult HIV seroprevalence greater than 5% is Haiti. In 9 countries (all in Southern Africa), the adult HIV seroprevalence rate is 15% or above. Sub-Saharan Africa thus bears the largest burden of the HIV/AIDS epidemic. However, certain countries in other regions are also badly affected by HIV, with an adult HIV seroprevalence of 1–5%, e.g. Cambodia, Myanmar and Thailand (South-East Asia) and Belize, Guatemala, Guyana, Haiti, Honduras, Panama, and Suriname (the Americas). HIV seroprevalence appears to be stabilizing in sub-Saharan Africa but is still increasing in some other large populations, e.g. in the Russian Federation. ROUTES OF TRANSMISSION. The HIV virus is found in blood, semen, vaginal secretions, breast milk, and saliva. Diagnosis by the ELISA test is presumptive; follow-up tests include Western blot and direct assessment of viral RNA. Transmission of HIV infection occurs by one of the following routes: 1. Sexual transmission. Sexual contact in the main mode of spread and constitutes 75% of all cases of HIV transmission. Most cases of AIDS occur in homosexual or bisexual males while heterosexual promiscuity seems to be the dominant mode of HIV infection in Africa and Asia. Other sexually transmitted diseases (STDs) may act as cofactors for spread of HIV, in particular gonorrhoeal and chlamydial infection. Transmission from male-to-male and male-to-female is more potent route than that from female-to-male. 65 2. Transmission via blood and blood products. This mode of transmission is the next commonest (25%) and occurs in 3 groups of high-risk populations: i) Intravenous drug abusers by sharing needles, syringes etc. comprise a large group in the US. ii) Haemophiliacs who have received large amounts of clotting factor concentrates from pooled blood components from multiple donors. iii) Recipients of HIV-infected blood and blood products who have received multiple transfusions of whole blood or components like platelets and plasma. 3. Perinatal transmission. HIV infection occurs from infected mother to the newborn during pregnancy transplacentally, or in immediate post-partum period through contamination with maternal blood, infected amniotic fluid or breast milk. 4. Occupational transmission. There have been a small number of health care workers (HCW), laboratory workers and those who have developed HIV infection by occupational exposure to HIV infected material. It is imperative that these workers follow guidelines for universal precautions which include disinfecting and sterilizing all reusable devices and use of bleaching solution for disinfecting all blood spillage. 5. Transmission by other body fluids. Although besides blood, HIV has been isolated and identified from a number of body fluids such as saliva, tears, sweat and urine, semen, vaginal secretions, cervical secretions, breast milk, CSF, synovial, pleural, peritoneal and pericardial fluid, there is no definite evidence that HIV transmission can occur by any of these fluids; isolated cases of such infection reported are in likelihood due to concomitant contamination with HIV-infected blood. AIDS high-risk categories: o Homosexual or bisexual men (75% of cases) The risk is apparently greater in anal intercourse. In Central Africa, the incidence in both sexes is about equal and is not higher in homosexual or bisexual men than in the general population. o Intravenous drug abusers (15% of cases). The virus is spread by sharing needles used by infected drug users. o Heterosexual partners of persons in high-risk groups (4% of cases). Sexual transmission from intravenous drug abusers is the major mode of entry of HIV into the heterosexual population. o Patients receiving multiple blood transfusions (2% of cases). Risk has been greatly diminished by screening donor blood for anti-HIV antibodies, HIV p24 antigen, and HIV-1 RNA. 66 Hemophiliacs (1% of cases). Most likely, the entire cohort of hemophiliacs who received factor VIII concentrates between 1981 and 1985 became infected with HIV. Since 1985, HIV screening and heat inactivation of HIV in factor VIII concentrates have become universal. o Infants of high-risk parents. Infection can be spread transplacentally or during delivery or breast-feeding. o PATHOGENESIS of HIV infection 1. Binding. The HIV virion expresses a cell surface protein gp120 with binding sites for the CD4 molecule on the surface of CD4+ T cells. The interaction of viral gp120 with cellular CD4 explains the affinity of HIV to CD4+ T cells. In addition, two recognition sites on gp120 for the co-receptors CCR5 and CXCR4 are involved in the entry of HIV into the cell. M-tropic (tropism to macrophages) HIV strains use CCR5, while T-tropic strains use the CXCR4 receptor. About 1% of whites are homozygous for asymptomatic major deletions in the CCR5 gene (the major mutation being a 32-base-pair deletion, causing a frame shift leading to a premature stop codon and a truncated, inactive protein product).These people remain uninfected with HIV even with extensive exposure to the agent. Even heterozygosity for the mutant CCR5 allele provides partial protection against HIV infection, and if infections do occur, they usually progress at a slower pace. Interestingly, the mutant allele is found in up to 20% of whites but is absent in blacks and Asians. o Other CD4+ cell types that are targets for HIV infection include monocytes, macrophages, dendritic cells, Langerhans cells, and microglial cells of the central nervous system (CNS), astrocytes and oligodendrocytes. Monocytes and macrophages may function as reservoirs for HIV and possibly as vehicles for viral entry into the CNS. HIV may infect neural cells directly by way of CD4 receptors or may compete (through the gp120 protein) for neural receptor sites for neuroleukin, a neural tissue growth factor. Neurons are not invaded by HIV but are affected due to attachment of gp120 and by release of cytokines by HIV-infected macrophages. 2. Internalisation. Gp120 of the virion combines with CD4 receptor, chemokine co-receptor (CCR) and gp41 glycoprotein resulting in internalization in the CD4+ T cell membrane. The viral capsid, genome and those enzymes are necessary for the early phase of the infectious cycle entering the cell. 3. Uncoating and viral DNA synthesis. After binding gp120 with CD4 and internalization of HIV into the cell, proviral DNA is synthesized by reverse transcription from genomic viral RNA. The resulting double-stranded DNA 67 version of the HIV-1 genome is called the provirus. This can remain in the cytoplasm, but when the cell divides the HIV DNA enters the nucleus. One critical function that this various RT lacks is an editing capacity. There is, consequently, a very high error rate in this whole process. 4. Viral integration. Proviral DNA is integrated into the host genome as a provirus. HIV-1 genomes therefore remain in the cell as long as the cell survives and are replicated along with host chromosomes. 5. Viral replication. Viral RNA is reproduced by transcriptional activation of the integrated HIV provirus, a process that, for example, for T cells, requires ―activation‖ of the infected cell plus certain inducible host transcription factors, especially nuclear factor-B (NF-B). 6. Viral dissemination. To complete its cycle, nascent virus is assembled in the cytoplasm just beneath the cell membrane and disseminated to other target cells. The mechanism by which HIV kills infected T-lymphocytes is still incompletely understood. Virus-induced apoptosis and autophagy may be activated. Other possibilities include immune destruction of the infected cells and the actions of secondary mediators such as cytokines. Whatever the mechanism(s), there is a clear association between increasing viral burden and declining CD4 -lymphocyte counts. Release of viral particles from infected host cell spreads the infection to new CD4+ host cells and produces viraemia. Through circulation, virus gains entry to the lymphoid tissues (lymph nodes, spleen) where it multiplies further, and they are the dominant site of virus reservoir rather than circulation. 7. Impact of HIV infection on other immune cells. HIV infects other cells of the host immune system and also affects non-infected lymphoid cells. o Infection with HIV results in the depletion of CD4+ T cells. The number of circulating lymphocytes is greatly decreased, and this decrease is accounted by the loss of CD4+ T cells. The CD4+:CD8+ ratio is also greatly reduced, often below 1.0. o The loss of CD4+ (helper) T cells causes failure of humoral and cell-mediated reactions. o Despite the inability to produce specific antibodies, patients with AIDS paradoxically demonstrate hypergammaglobulinemia from polyclonal B-cell activation. o Immune resistance to HIV infection involves B-cell activation (with lymph node hyperplasia) and killing by NK-cells infected T-cells by apoptosis, but these do not eliminate the infection, which progressively destroys the cellmediated immune system by destruction of T-helper cells faster than they can be renewed. 68 o Over time, the HIV in a person mutates from M-tropic to a T-tropic strain. This is significant because T-tropic strains are more virulent and cause severe T-cells cytolysis. NATURAL HISTORY. HIV infection progresses from an early acute syndrome to a prolonged asymptomatic state to the advanced disease. Thus there are different clinical manifestations at different stages. Generally, in an immunocompetent host, the biologic course passes through the following phases: Acute retroviral syndrome. (3-12 weeks). Entry of HIV into the body is heralded by the following sequence of events: High levels of plasma viruses due to replication of the virus. Virus-specific immune response with formation of anti-HIV antibodies (seroconversion) 3-6 weeks after the initial exposure to HIV. Initially, sudden marked reduction in CD4+ T cells (helper T cells) followed by return to normal levels. Rise in CD8+ T cells Appearance of self-limited non-specific acute viral illness (flu-like or infectious mononucleosis-like) in 50-70% of adults 3-6 weeks within the initial infection. Manifestations include: sore throat, fever, myalgia, skin rash, and sometimes, aseptic meningitis. Less commonly, patients present with neurologic symptoms that suggest encephalitis, aseptic meningitis or a neuropathy. These symptoms resolve spontaneously in 2-3 weeks. Asymptomatic HIV infection In adults, there is a long, variable, latent period from HIV infection to the onset of HIV-related disease and AIDS. A person infected with HIV may be asymptomatic for 10 years or more. The period of asymptomatic infection is shorter in children than in adults. A few infants become ill in the first few weeks of life. The majority of children become ill before 2 years of age. A few children remain well for several years. Persistent generalized lymphadenopathy is palpable lymph node enlargement in two or more extrainguinal sites, persisting for more than 3 months in a person infected with HIV. The disorder develops either as part of the acute HIV syndrome or within a few months of seroconversion. The most common sites of involvement are the axillary, inguinal and posterior cervical nodes, although almost any group of lymph nodes can be affected. Many cells within the affected lymph nodes, especially follicular dendritic cells, harbor actively replicating virus. Biopsy reveals reactive changes with follicular hyperplasia. 69 Progression from HIV infection to HIV-related disease. Almost all (if not all) HIV-infected people, if untreated, will ultimately develop HIV-related disease and AIDS. Some HIV-infected individuals progress more quickly than the others. The rate of progression depends on virus and host characteristics. Virus characteristics include type and subtype: HIV-1 and certain HIV-1 subtypes may cause faster progression. Host characteristics that may cause faster progression include: age less than 5 years or more than 40 years; concurrent infections; genetic factors. Advancing immunosuppression and AIDS. The last stage, defined as AIDS, is marked by HIV infection complicated by specified secondary opportunistic infection or malignant neoplasms. As HIV infection progresses and immunity declines, patients become more susceptible to infections. These include TB, pneumonia, recurrent fungal infections of the skin and oropharynx, and herpes zoster. These infections can occur at any stage of progression of HIV infection and immunosuppression. Some patients may develop constitutional symptoms (unexplained fever and weight loss), previously known as "AIDS-related complex" (ARC). Some patients develop chronic diarrhea with weight loss, often known as "slim disease". Certain specific HIV-related diseases occur predominantly with severe immunosuppression. These include certain opportunistic infections (e.g. cryptococcal meningitis) and certain tumors (e.g. Kaposi sarcoma). At this late stage, without specific therapy, patients usually die in less than 2 years. This late stage is sometimes known as "full-blown AIDS". WHO CLINICAL STAGING OF HIV/AIDS FOR ADULTS AND ADOLESCENTS Asymptomatic Primary Acute retroviral syndrome HIV infection Asymptomatic Clinical Persistent generalized lymphadenopathy (PGL) stage 1 Moderate unexplained weight loss (<10% of presumed or measured Clinical body weight) stage 2 Recurrent respiratory tract infections (RTIs, sinusitis, bronchitis, otitis media, pharyngitis) Herpes zoster Angular cheilitis Recurrent oral ulcerations Papular pruritic eruptions Seborrhoeic dermatitis 70 Clinical stage 3 Clinical stage 4 Mycosis of nails Severe weight loss (>10% of presumed or measured body weight) Unexplained chronic diarrhea longer than one month Unexplained persistent fever (intermittent or constant for longer than one month) Oral candidiasis Oral hairy leukoplakia Pulmonary tuberculosis (TB) diagnosed in last two years Severe presumed bacterial infections (e.g. pneumonia, empyema, pyomyositis, bone or joint infection, meningitis, bacteraemia) Acute necrotizing ulcerative stomatitis, gingivitis or periodontitis Conditions where confirmatory diagnostic testing is necessary Unexplained anaemia (< 8 g/dl), and or neutropenia (<500/mm3) and or thrombocytopenia (<50 000/ mm3) for more than one month HIV wasting syndrome Pneumocystis pneumonia Recurrent severe or radiological bacterial pneumonia Chronic herpes simplex infection (orolabial, genital or anorectal of more than one month‘s duration) Oesophageal candidiasis Extrapulmonary TB Kaposi‘s sarcoma Central nervous system (CNS) toxoplasmosis HIV encephalopathy Conditions where confirmatory diagnostic testing is necessary: Extrapulmonary cryptococcosis including meningitis Disseminated non-tuberculous mycobacteria infection Progressive multifocal leukoencephalopathy (PML) Candida of trachea, bronchi or lungs Cryptosporidiosis Isosporiasis Visceral herpes simplex infection Cytomegalovirus (CMV) infection (retinitis or of an organ other than liver, spleen or lymph nodes) Any disseminated mycosis (e.g. histoplasmosis, coccidiomycosis, penicilliosis) Recurrent non-typhoidal salmonella septicaemia 71 Lymphoma (cerebral or B cell non-Hodgkin) Invasive cervical carcinoma Visceral leishmaniasis Russian Classification of HIV Infection (V. I. Pokrovsky, 2001) 1. Incubation stage 2. Stage of primary manifestations A. Asymptomatic B. Acute HIV infection without secondary diseases. C. Acute HIV infection with secondary diseases. 3. Latent stage 4. Stage of secondary diseases 4A. Weight loss less than 10%; fungal, viral, or bacterial lesions of the skin and mucous membranes; herpes zoster; recurrent pharyngitis, sinusitis. Phases: Progression, Remission 4B. Weight loss more than 10%: unexplained diarrhea or fever lasting more than one month; hairy leukoplakia; pulmonary tuberculosis; recurrent or persistent viral, bacterial, fungal, or protozoal pathology of the internal organs; Phases: Progression, Remission 4C. Cachexia; generalized bacterial, viral, fungal, protozoal, and parasitic diseases; pneumocystis pneumonia; esophageal, bronchial, and pulmonary candidiasis; extrapulmonary tuberculosis; atypical mycobacterioses; disseminated Kaposi's sarcoma; lesions of the central nervous system of varied etiology. Phases: Progression, Remission 5. Terminal stage. PATHOLOGY AND CLINICAL FEATURES: Clinical characteristics of HIV-infection: During some time the number of CD4 T-cells starts to decrease. Patients generally remain asymptomatic until the CD4-lymphocyte count falls below 500/L. Then, nonspecific constitutional symptoms may appear, along with opportunistic infections. As CD4 T-cells fall below 350/L, patients become much more susceptible to primary or reactivation of Mycobacterium tuberculosis, which may progress rapidly to severe disease or death. Once CD4 levels are under 150/L and CD4:CD8 ratios less than 0.8, the disease progresses rapidly. A variety of bacteria, viruses, fungi and protozoa attack the immunocompromised patient. Severe immunodeficiency manifested by opportunistic infection with organisms such as Pneumocystis jiroveci (Pneumocystis carinii), cytomegalovirus, Mucor species, 72 and typical and atypical mycobacteria such as Mycobacterium aviumintracellulare; other opportunistic infections often found include Candida, Cryptosporidium, Coccidioides, Cryptococcus, Toxoplasma, Histoplasma, and Giardia infections. Increased incidence of malignancy, particularly multifocal Kaposi sarcoma, an otherwise rare lesion that in AIDS is almost entirely confined to the homosexual male population, and B-cell non-Hodgkin lymphoma; an increased incidence of Hodgkin disease and hepatocellular carcinoma also occurs. This lesion is associated with human herpesvirus 8 (HHV-8), also called Kaposi sarcoma herpesvirus (KSHV). Central and peripheral nervous system manifestations occur due to opportunistic infections, CNS tumors, or direct neural infection with HIV. The main HIV-associated disease Viral Infections Cytomegalovirus: Cytomegalovirus (CMV) is a DNA herpes virus and latently infects most people. It is acquired transplacentally, during infancy, by respiratory droplet infection and via sexual intercourse. It reactivates in states of immunosuppression – particularly HIV/AIDS – and also in transplant recipient patients. By infecting the endothelial and epithelial cells it causes cytolytic cell damage and focal necrosis. Common presentations in HIV/AIDS are a pneumonitis, intestinal lesions with diarrhoea, confusional states from encephalitis and, importantly, visual defects. Retinal CMV infection is a significant cause of blindness in HIV disease, and is the reason why many patients are given prophylactic antiherpes virus therapy. Morphologically, the nucleated layers of the retinal epithelium show the characteristic CMV nuclear and cytoplasmic inclusions, with necrosis. CMV infection is diagnosed by finding the viral inclusions in biopsies and virological identification in samples of blood and cerebrospinal fluid (CSF). Herpes Simplex: This is representative of the large group of human herpes DNA viruses which includes herpes simplex virus (HSV) types 1 and 2, herpes zoster, CMV, Epstein–Barr virus (EBV) and HHV8 (the cause of Kaposi‘s sarcoma). HSV is transmitted by direct contact with infected lesions, mainly from sexual intercourse. It primarily affects the genital skin and mucosae, but it is also neurotropic, and can disseminate to internal organs. Whether or not there is clinical primary infection, latent infection is thereafter lifelong, and HSV reactivates in immunosuppression. The skin and mucosal lesions are painful and erosive. Typically, they are on the penis, vulva and around the mouth (‗cold sore‘). Pathologically, the epithelial cells contain characteristic nuclear inclusions of virus, associated with cytolytic necrosis and inflammation. 73 Similar pathology is seen in the skin lesions of chicken pox, caused by herpes zoster. In HIV/AIDS, severe HSV skin and mucosal ulceration can occur, involving the genitalia, mouth and oesophagus. It can also cause necrotizing encephalitis and ulcerating conjunctival keratitis; both these lesions also occur in people without evident immunosuppression. JC Virus: This is a papovavirus that everyone acquires as a latent infection in childhood. In late HIV disease, it can reactivate, and cause a characteristic cytolytic infection of brain oligodendrocytes. This results in white matter necrosis. Molluscum Contagiosum: This is a poxvirus infection that produces multiple small white nodules on the skin. It is normally common in childhood, and is more florid in people with HIV. Bacterial Infections Streptococcal infection causes both pneumonia and septicaemia; non-typhoid Salmonella infections cause enteritis and septic shock. HIV-associated tuberculosis. HIV-infected individuals have a very high incidence of tuberculosis all over the world. Vice-versa, rate of HIV infection in patients of tuberculosis is very high. Moreover, HIV-infected individual on acquiring the infection with tubercle bacilli develops active disease rapidly (within few weeks) rather than after months or years. Pulmonary tuberculosis in HIV presents in a typical manner. However, it is more often sputum smear negative but often culture positive. Extra-pulmonary tuberculosis is more common in HIV disease and manifests commonly by involving lymph nodes, pleura, pericardium, and tuberculous meningitis. Infection with M. avium-intracellulare (avian or bird strain) is common in patients with HIV/AIDS. Fungal Infections Pneumocystis jirovecii (carinii): This is a ubiquitous fungus in the environment (although it was once classified as a protozoan parasite) to which everyone is exposed. Like many infections of very low virulent potential, it causes disease only in those significantly immunosuppressed. Whilst it is now seen mainly in those with HIV infection (adults and children) and transplant patients (especially renal transplant), there have been epidemics in infancy that are related to malnutrition. The infection can present rapidly with shortness of breath and prostration. Chest Xradiography shows fluffy fine shadows in the perihilar zones or throughout the lung fields. On pathological examination, the lung in Pneumocystis pneumonia is solid, pale brown and dry. Microscopically, the alveoli are filled with masses of cysts, thus preventing gas exchange. The cysts are 3–4 μm in diameter, and contain small 74 nuclei. There is often a mild interstitial lymphoplasmacytic infiltrate (interstitial pneumonitis). Aided by chemotherapy, macrophages phagocytose the fungi to clear the alveoli. However, if the infection persists or returns, there is progressive intra-alveolar organization and interstitial fibrosis. The diagnosis of Pneumocystis pneumonia is made by identifying the organisms in sputum or bronchial lavage specimens, or by lung biopsy. Standard treatment is with co-trimoxazole, which enables the lung to return to normal. However, in those with continuing immunosuppression, prophylactic therapy needs to be maintained. If and when pneumocystosis returns, it tends to cause progressive lung damage, with fibrosis and even cavitation. Cryptococcus neoformans: This fungus is present globally in bird droppings and the soil. Infection is acquired by inhalation, but clinical disease is uncommon in people who are not immunocompromised. The primary lesion is a pneumonia: it may be localized (a ‗cryptococcoma‘ that can present as a mass on chest X-radiography) or, more commonly, as a diffuse process causing breathlessness. HIV-infected patients are prone to developing cryptococcal meningo-encephalitis, presenting with confusion or focal neurological signs. Pathologically, in HIV disease, the fungus elicits a minimal cellular reaction, so within the consolidated lung one sees alveoli filled with yeast. The brain shows milky, thick meninges and mucoid holes in the grey and white matter (resembling Swiss cheese). Histologically, the fungal yeasts have a mucoid capsule; they lie in unactivated macrophages or free in parenchyma and spaces around vessels. Diagnosis is based on seeing the yeasts in tissue samples (e.g. CSF or biopsies), supported by antibody detection of antigen in blood or CSF (the CrAg test). Modern antifungal therapy can clear the disease, but it always recurs if prophylactic therapy is not continued. Protozoal Infections Toxoplasma gondii - Disease of the brain and central nervous system Cryptosporidium parvum is a gut parasite of cattle that can be transmitted to man via contaminated water. In non-immunocompromised people it causes self-limiting diarrhoea. However, in immunocompromised patients, chronic choleralike diarrhoea results, with malabsorption. C. parvum infects the small and large bowel mucosae, the parasites residing in large numbers just within the enterocyte surface membrane;small intestinal villi are blunted, and there is enteritis. Several species of microsporidia (e.g. Enterocytozoon bieneusi) have been newly described in people with severe HIV-associated immunosuppression, and globally are a common cause of diarrhoea. The parasites reside within the enterocyte cytoplasm. 75 As with Cryptosporidium, the exact pathogenesis of the diarrhoeal disease is unclear. Diagnosis of both infections is by finding the parasites in faeces, or by gut biopsy. No specific therapy is available, but anti-HIV chemotherapy often eradicates the infections. HIV-associated Tumours Kaposi‘s sarcoma (KS) – which was first described in 1872 – is a peculiar proliferation of endothelial cells affecting the skin, mucosal surfaces, lymph nodes and many internal organs. Although HIV infection greatly increases the frequency of KS, it is not a necessary factor in the lesion‘s development. In 1994 a virus, human herpesvirus type 8 (HHV8), was found to be the significant factor; this is globally distributed and is transmitted by sexual intercourse, and vertically to children. In all organs, KS develops as a red flat lesion (macule on the skin), and then thickens to plaques and infiltrative red nodules. It behaves as a space-occupying lesion and causes significant local oedema (e.g. in the subcutis and lung). Histologically, the mature lesion is a spindle-cell tumour with red blood cells between the endothelial tumour cells. CLASSIFICATION. Presently, four forms of Kaposi‘s sarcoma are described: 1. Classic (European) Kaposi’s sarcoma. This is the form which was first described by Kaposi. It is more common in men over 60 years of age of Eastern European descent. The disease is slowly growing and appears as multiple, small, purple, dome-shaped nodules or plaques in the skin, especially on the legs. Involvement of visceral organs occurs in about 10% cases after many years. 2. African (Endemic) Kaposi’s sarcoma. This form is common in equatorial Africa. It is so common in Uganda that it comprises 9% of all malignant tumours in men. It is found in a younger age, especially in boys and in young men and has a more aggressive course than the classic form. The disease begins in the skin but grows rapidly to involve other tissues, especially lymph nodes and the gut. 3. Epidemic (AIDS-associated) Kaposi’s sarcoma. This form is seen in about 30% cases of AIDS, especially in young male homosexuals than the other high-risk groups. The cutaneous lesions are not localized to lower legs but are more extensively distributed involving mucous membranes, lymph nodes and internal organs early in the course of the disease. 4. Kaposi’s sarcoma in transplant cases. This form is associated with recipients of renal transplants who have been administered immunosuppressive therapy for a long time. The lesions may be localised to the skin or may have a widespread systemic involvement. 76 PATHOGENESIS. Pathogenesis of Kaposi‘s sarcoma is complex. It is an opportunistic neoplasm in immunosuppressed patients which has excessive proliferation of spindle cells of vascular origin having features of both endothelium and smooth muscle cells. Epidemiological studies have suggested a viral association implicating HIV and human herpesvirus 8 (HSV 8, also called Kaposi‘s sarcoma-associated herpesvirus or KSHV). Occurrence of Kaposi‘s sarcoma involves interplay of HIV-1 infection, HHV-8 infection, activation of the immune system and secretion of cytokines (IL-6, TNF-α, GM-CSF, basic fibroblast factor, and oncostain M). Higher incidence of Kaposi‘s sarcoma in male homosexuals is explained by increased secretion of cytokines by their activated immune system. Defective immunoregulation plays a role in its pathogenesis is further substantiated by observation of second malignancy (e.g. leukaemia, lymphoma and myeloma) in about one-third of patients with Kaposi‘s sarcoma. MORPHOLOGIC FEATURES. Pathologically, all forms of Kaposi‘s sarcoma are similar. Grossly, the lesions in the skin, gut and other organs form prominent, irregular, purple, dome-shaped plaques or nodules. Histologically, the changes are nonspecific in the early patch stage and more characteristic in the late nodular stage. Early patch stage: There are irregular vascular spaces separated by interstitial inflammatory cells and extravasated blood and haemosiderin. Late nodular stage: There are slit-like vascular spaces containing red blood cells and separated by spindleshaped, plump tumour cells. These spindle-shaped tumour cells are probably of endothelial origin. CLINICAL COURSE. The clinical course and biologic behaviour of Kaposi‘s sarcoma is quite variable. The classic form of Kaposi‘s sarcoma is largely confined to skin and the course is generally slow and insidious with long survival. The endemic (African) and epidemic (AIDS-associated) Kaposi‘s sarcoma, on the other hand, has a rapidly progressive course, often with widespread cutaneous as well as visceral involvement, and high mortality. Lymphoma: HIV-associated lymphomas comprise B-cell, high-grade tumours, located in extranodal sites. They are 100-fold more common in HIV-infected people than in the normal population, and many are associated with EBV infection. Cerebral lymphoma presents with focal neurological signs and confusion; pathologically, there are one or more tumour masses within the brain, the malignant cells spreading out from the blood vessel walls. Elsewhere, tumours present as oral and intestinal ulcerating lesions, and with pulmonary and retroperitoneal masses. A common histological pattern is high-grade B-cell lymphoma similar to that of Burkitt lymphoma. 77 Mucosal Squamous Cell Neoplasms: Intraepithelial and invasive squamous cell neoplasms of the cervix and anus are aetiologically associated with human papilloma virus (HPV) infections. People who are coinfected with HIV have a significant risk of developing dysplasia and in-situ carcinoma at these sites, which are often detected by screening. However, invasive malignant lesions are less frequent than expected. In central Africa, conjunctival carcinoma (associated with UV light exposure) is also more common in those with HIV infection. Neurological Disease in HIV/AIDS (AIDS-Dementia Complex) HIV-associated dementia is a late phenomenon. It appears to be caused by the cortical neuronal loss and abnormalities of the dendritic connections. Morphologically, there is cerebral atrophy, and the characteristic HIV encephalitis comprises multiple nodules of infected microglia and microglial giant cells in the white matter. By secreting toxic cytokines, these lesions also affect the neural function. One major clinical feature of this entity is the occurrence of dementia i.e. fall in the cognitive ability of the individual compared to the previous level. The condition is believed to be the result of direct effect of HIV on the CNS. Clinically, the disease develops in about 25% cases of AIDS while autopsy studies reveal presence of HIV-encephalopathy in 80-90% cases of AIDS. PATHOLOGY: HIV-associated dementia is characterized by mild cerebral atrophy, dilation of the lateral ventricles and slight prominence of gyri and sulci. Histologic changes are usually in the subcortical gray and white matter. Histologically, the changes are more in subcortical area of the brain and consist of gliosis, multinucleate giant cell encephalitis, and vacuolar myelopathy. Vacuolar myelopathy is another disorder attributed to HIV infection, although it is less frequent than encephalopathy. It is characterized by marked vacuolation of the posterior and lateral columns, principally at the thoracic level of the spinal cord. Ataxia and spastic paraparesis dominate the clinical presentation. Renal Disease in HIV/AIDS Many opportunistic diseases can affect the kidney (e.g. cryptococcosis, CMV infection of the tubules, pyaemic bacterial infection, tuberculosis, lymphoma), but one specific entity is important – HIV-associated nephropathy (HIVAN). Clinically, this condition presents with renal failure and nephrotic syndrome. Pathologically, it is characterized by focal segmental glomerulosclerosis (FSGS) and interstitial inflammation with tubular atrophy and dilatation. It appears to be caused directly by HIV, and is significant because it is an ethnically-restricted opportunistic disease in HIV/AIDS: it occurs only in ople of African descent. Paediatric HIV Disease 78 This is common in many poor parts of the world since, without antiretroviral chemotherapy during pregnancy and delivery, about 25% of fetuses are infected if the mother is HIV-infected. The clinical and pathological features are listed in Table 19.16.Without specific treatment, the mortality in children is 50% by the age of 3 years, and few survive 5 years. References: 1. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2009, 17:67 http://www.sjtrem.com/content/17/1/67 examples of Recently Discovered and Emerging Infection The Systemic Inflammatory Response Syndrome (SIRS) in acutely hospitalised medical patients: a cohort study. Pål Comstedt, Merete Storgaard and Annmarie T Lassen 2. Surviving Sepsis Campaign International Guidelines for Management of 3. Severe Sepsis and Septic Shock: 2012 - Critical Care Medicine February 2013 Volume 41 • Number 2 4. American public health association. Control of communicable diseases in man. Washington DC:American public health association; 1995. 5. Noah DL, Sovel AL, Ostroff SM, Kildew JA. Biological warfare training: infectious disease outbreak differentiation criteria. Mil Med 1998;163:198201. 6. Tucker JB. National health and medical services response to incidents of chemical and biological terrorism. JAMA 1997;278:362-8. 7. Pile JC, Malone JD, Eitzen EM, Friedlander AM. Anthrax as a potential biological warfare agent. Arch Intern Med 1998;158:429-34. 8. ―Exploring. HIV Infection and AIDS: An Overview.‖ National Institute of Allergy and Infectious Diseases. March 2005. 9. <www.niaid.nih.gov/factsheets/hivinf.htm>. 10.―Human Immumodeficiency Virus (HIV) and Acquired Immunodeficiency Syndrome (AIDS)‖ Guide to Surveillance, Reporting and Control 399-405 Massachusetts Department of Public Health, Bureau of Communicable Disease Control. June 2006 11.World Health Organization. TB/HIV A CLINICAL MANUAL Second edition Geneva, 2004 (WHO/HTM/TB/2004.329). 79 NOTES: 80 81 Учебное пособие Корнева Юлия Сергеевна, Скробут Лариса Иосифовна, PATHOLOGY OF INFECTIOUS DISEASES под редакцией д.м.н., проф. Доросевича Александра Евдокимовича 82 Tutorial Korneva Yulia Sergeevna, Scrobut Larisa Iosiphovna, PATHOLOGY OF INFECTIOUS DISEASES under the editorship of MD, Phd Dorosevich Alexander Evdokimovich 83 84