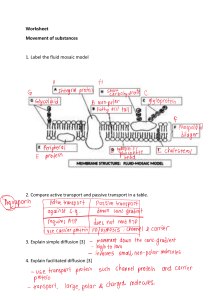
KMK20403 Laboratory Report 2023/2024 KMK20403 Laboratory Report 2023/2024 OBJECTIVES : 1. To demonstrate the liquid diffusion process under different mixing rates 2. To determine liquid diffusion coefficient based on effect of mixing rate. INTRODUCTION: The purpose of the SOLTEQ® Liquid Diffusion Coefficient Apparatus (Model: BP 09) is to allow students to conduct experiments involving the measurement of sodium chloride solution diffusivity in dried water. Distilled water is submerged in a diffusion cell containing a NaCl solution with a known concentration. a magnetic to track the diffusion process over time, a stirrer and a conductivity meter are included. The student can calculate the NaCl solution's liquid diffusivity by creating a conductivity versus time plot. Figure 1 and 2 shows SOLTEQ® Liquid Diffusion Coefficient Apparatus (Model: BP 09) Diffusion is a microscopic process of mixing brought about by the molecular motion of the particles. Its importance stems from the fact that it is a slow process in liquids. It can be the phase that determines the rate of many mass transfer processes, including extraction, distillation, and industrial reactions involving porous catalysts. For example, it regulates how food releases flavor. For this reason, understanding diffusion rates is crucial when designing process equipment. Fick's Law can be extended to multicomponent systems to explain KMK20403 Laboratory Report 2023/2024 molecular diffusion in multicomponent liquids, according to common models. For binary liquid systems, diffusion is described phenomenologically by Fick's law. The term "molecular diffusion" refers to the random, irregular movements that individual molecules in a mixture cause due to their thermal energy. However, gradients in temperature, pressure, concentration, and external force fields can also cause it. When a concentration gradient is present, the resulting net diffusion flux is down the gradient, or from regions of higher to lower concentration, until the system reaches uniformity. While concentration gradients are the most common cause of diffusion, other possible causes include activity gradients, such as those found in reverse osmosis, pressure gradients, temperature gradients, and the application of an external force field, such as those found in centrifuges. Thermal diffusion is the movement of molecules caused by temperature; forced diffusion is the movement of molecules caused by an external field. Diffusion is not limited to the movement of molecules across a layer of solid or liquid that is stagnant. It also occurs when mixtures of fluids with various compositions are made. Mass transfer, which is often the initial stage of mixing, is brought on by the turbulent flow's eddy motion. We refer to this as eddy diffusion. Molecular diffusion occurs both inside and between the minuscule eddies in the second step. Sometimes the diffusion process is accompanied by bulk flow of the mixture in a direction parallel to the direction of diffusion METHODOLOGY: KMK20403 Laboratory Report 2023/2024 RESULTS: KMK20403 Laboratory Report 2023/2024 Graph 1: Time (ks) against Conductivity k (μs) at 1M Sodium Chloride solution KMK20403 Laboratory Report 2023/2024 KMK20403 Laboratory Report 2023/2024 Graph 2: Time (ks) vs Conductivity, k (μs) at 2M NaCl solution KMK20403 Laboratory Report 2023/2024 Graph 3: Time (ks) vs Conductivity, k(μs) at 4M NaCL solution KMK20403 Laboratory Report 2023/2024 DISCUSSION: CONCLUSION: The experiment investigated the impact of solution concentration on the liquid diffusion coefficient. It demonstrated that higher solution concentrations resulted in slower diffusion rates, while lower concentrations led to faster diffusion. The diffusion coefficient decreased as concentration increased. This study highlights the importance of concentration gradients in influencing diffusion and provides practical insights for controlling and optimizing diffusion processes in various fields. KMK20403 Laboratory Report 2023/2024 REFERENCES: 1. Long, A. A. (n.d.). EXPERIMENT 3 LIQUID DIFFUSION APPARATUS. Scribd. https://www.scribd.com/doc/151016857/EXPERIMENT-3-LIQUID-DIFFUSIONAPPARATUS 2. Jafni, A. (n.d.). Liquid diffusion. Scribd. https://www.scribd.com/doc/167378844/Liquid-Diffusion 3.