Kinetic theory of gases student PART 1
advertisement

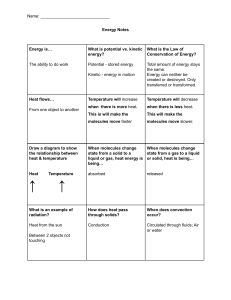
CONTENTS 1. INTRODUCTION MOLECULAR NATURE OF MATTER DALTONS ATOMIC THEORY GAY LUSSAC’S LAW 2. BEHAVIOUR OF GASES GASES MOLE: Boltzmann constant, Avogadro number PERFECT GAS EQUATION DALTON’S LAW OF PARTIAL PRESSURES 3. KINETIC THEORY OF AN IDEAL GAS PRESSURE OF AN IDEAL GAS KINETIC INTERPRETATION OF TEMPERATURE RMS SPEED (Vrms) AVOGADRO’S HYPOTHESIS 1. INTRODUCTION Kinetic theory explains the behaviour of gases based on the idea that the gas consists of rapidly moving atoms or molecules. MOLECULAR NATURE OF MATTER Atomic Hypothesis: All things are made of atoms (little particles) that move around in perpetual motion, attracting each other when they are a little distance apart. DALTONS ATOMIC THEORY: The first law says that any given compound has a fixed proportion by mass of its constituents. The second law says that when two elements form more than one compound, for a fixed mass of one element, the masses of the other elements are in ratio of small integers. GAY LUSSAC’S LAW (Law of pressure): When the volume is constant, the pressure of a given mass of a gas is directly proportional to its absolute temperature. Pt P0 t(°C) – 273.15 O 2. BEHAVIOUR OF GASES GASES: The gas is full of activity and the equilibrium is a dynamic one. In dynamic equilibrium, molecules collide and change their speeds during the collision. Only the average properties are constant. Gases at low pressures and high temperatures much above that at which they liquefy (or solidify) approximately satisfy a simple relation between their pressure, temperature and volume given by PV = KT For a given sample of the gas. Here, K is a constant for the given sample but varies with the volume of the gas. If we now bring in the idea of atoms or molecules then K is proportional to the number of molecules, (say) N in the sample. We can write K = N kB . Observation tells us that this kB is same for all gases. It is called as Boltzmann constant. P1V1 N 1 T1 = P2V2 N 2 T2 = constant = kB = 1.38 × 10–23 J K–1 Avogadro number = NA = number of molecules in 22.4 litres of any gas is 6.02 × 1023. MOLE: The mass of 22.4 litres of any gas is equal to its molecular weight in grams at S.T.P. This amount of substance is called a mole. The number of moles μ = number of molecules (N) NA Mass = Molecular mass = R = NA kB is a universal constant = 8.314 J mol–1K–1. The PERFECT GAS EQUATION can be written as PV = NT K B vloume 22.4 lit N= μNA PV = μNA T K B PV = μ RT A gas that satisfies PV = μ RT exactly at all pressures and temperatures is defined to be an ideal gas. P= ρ RT Mo where ρ is the mass density of the gas Finally, consider a mixture of non-interacting ideal gases: μ1 moles of gas 1, μ2 moles of gas 2, etc. in a vessel of volume V at temperature T and pressure P. It is then found that the equation of state of the mixture is PV = ( μ1 + μ2 +… ) RT RT RT P = μ1 V + μ2 V +… P = P1 + P2 + ........... Experimental P-V curves (solid lines) for steam at three temperatures compared with Boyle’s law (dotted lines). P is in units of 22 atm and V in units of 0.09 litres Experimental T-V curves (solid lines) for CO2 at three pressures compared with Charles’ law (dotted lines). T is in units of 300 K and V in units of 0.13 litres Real gases approach ideal gas behaviour at low pressures and high temperatures Sample Problems based on Ideal gas equation Problem 1. Solution : (c) Problem 2. A gas at 27°C has a volume V and pressure P. On heating its pressure is doubled and volume becomes three times. The resulting temperature of the gas will be (a) 1800°C (b) 162°C (c) 1527°C (d) 600°C P V 2 P 3V T From ideal gas equation PV RT we get 2 2 2 1 1 6 T1 P1 V1 P1 V1 T2 6 T1 6 300 1800 K 1527 C. A balloon contains 500 m 3 of helium at 27°C and 1 atmosphere pressure. The volume of the helium at – 3°C temperature and 0.5 atmosphere pressure will be 3 (a) 500 m 3 (b) 700 m 3 (c) 900 m 3 (d) 1000 m V2 T2 P1 270 1 9 9 V2 500 900 m 3 5 V1 T1 P2 300 0 .5 5 Solution : (c) From PV RT we get Problem 3. When volume of system is increased two times and temperature is decreased half of its initial temperature, then pressure becomes (a) 2 times (b) 4 times (c) 1 / 4 times (d) 1 / 2 times T V T / 2 V1 1 P P From PV RT we get 2 2 1 1 P2 1 4 P1 T1 V2 T1 2 V1 4 Solution : (c) Problem 4. Solution : (d) The pressure P, volume V and temperature T of a gas in the jar A and the other gas in the jar B at pressure 2P, volume V/4 and temperature 2T, then the ratio of the number of molecules in the jar A and B will be [AIIMS 1982] (a) 1 : 1 (b) 1 : 2 (c) 2 : 1 (d) 4 : 1 N RT where N = Number of molecule, NA = Avogadro number Ideal gas equation PV RT NA N 1 P1 V1 T2 P V 2T 4 . N 2 P2 V2 T1 2 P V /4 T 1 DALTON’S LAW OF PARTIAL PRESSURES: The total pressure of a mixture of ideal gases is the sum of partial pressures. 3. KINETIC THEORY OF AN IDEAL GAS P = P1 + P2 + ........... The molecules collide incessantly against each other or with the walls and change their velocities. The collisions are considered to be elastic. PRESSURE OF AN IDEAL GAS Consider a gas enclosed in a cube of side l. A molecule with velocity (vx, vy, vz) hits the planar wall parallel to yz plane. Since the collision is elastic, the molecule rebounds with the same velocity; its y and z components of velocity do not change in the collision but the x-component reverses sign. That is, the velocity after collision is (-vx, vy, vz). Elastic collision of a gas molecule with the wall of the container The change in momentum of the molecule is : –mvx – (mvx) = – 2mvx. In a small time interval Δt, a molecule with x-component of velocity vx will hit the wall if it is within the distance vx Δt from the wall. But, on the average, half of these are moving towards the wall and the other half away from the wall. Thus the 1 number of molecules with velocity (vx, vy, vz) hitting the wall in time Δt is 2A vx Δt n where n is the number of molecules per unit volume. The total momentum transferred to the wall by these molecules in time Δt is : Q = (2mvx) (½ n A vx Δt) = mn A vx 2 Δt The force on the wall is the rate of momentum transfer = F = Q/Δt = mn A vx 2 F pressure is force per unit area : P = A = mn vx 2 Actually, all molecules in a gas do not have the same velocity; there is a distribution in velocities. Total pressure is obtained by summing over the contribution due to all groups: P = n m vx 2 Where vx 2 is the average of vx 2 i.e.,vx 2 = If the gas is isotropic, v x 1 2 +v x 2 2 +v x 3 2 +⋯v xn 2 vx 2 = vy 2 = vz 2 n vx 2 + vy 2 + vz 2 = v 2 1 1 vx 2 = vy 2 = vz 2 = 3 vx 2 + vy 2 + vz 2 = 3 v 2 v 2 +v 2 2 +v 3 2 +⋯v n 2 Where v is the speed and v 2 denotes the mean of the squared speed, v 2 = 1 Root mean square speed (vrms) = v 2 = n v 1 2 +v 2 2 +v 3 2 +⋯v n 2 n 1 1 P = mn 3 v 2 = 3 mnv 2 KINETIC INTERPRETATION OF TEMPERATURE 1 1 PV = mn V 3 v 2 = 3 N mv 2 PV = 2 3 [∵ N = Vn] 1 N mv 2 2 Since the internal energy E of an ideal gas is purely kinetic. E=N 1 2 PV = mv 2 2 E 3 Combining above equation with the ideal gas Eq. PV = NT K B we get, 2 3 E = NT K B 3 E = N KB T 2 Thus, internal energy of an ideal gas depends only on temperature, not on pressure or volume. The average kinetic energy of a molecule is proportional to the absolute temperature of the gas; it is independent of pressure, volume or the nature of the ideal gas. Sample Problems based on Pressure & Kinetic energy Problem 1. A cylinder of capacity 20 litres is filled with H 2 gas. The total average kinetic energy of translatory motion of its 5 molecules is 1 .5 10 J . The pressure of hydrogen in the cylinder is 6 2 (a) 2 10 N / m Solution : (d) 6 2 (b) 3 10 N / m 6 2 (c) 4 10 N / m 5 Kinetic energy E = 1 .5 10 J , volume V = 20 litre = 20 10 3 m 3 6 2 (d) 5 10 N / m Pressure Problem 2. 2 E 2 1 .5 10 5 5 10 6 N /m 2 . 3 V 3 20 10 3 A cylinder of capacity 20 litres is filled with H 2 gas. The total average kinetic energy of translatory motion of its 5 molecules is 1 .5 10 J . The pressure of hydrogen in the cylinder is 6 2 (a) 2 10 N / m Solution : (d) 6 2 (c) 4 10 N / m 6 2 (d) 5 10 N / m 5 Kinetic energy E = 1 .5 10 J , volume V = 20 litre = 20 10 3 m 3 Pressure Problem 3. 6 2 (b) 3 10 N / m 2 E 2 1 .5 10 5 5 10 6 N /m 2 . 3 V 3 20 10 3 The kinetic energy of one mole of a gas at normal temperature and pressure is (R = 8.31 J/mole-K) (a) 0.56 10 4 J (c) 2 .7 10 2 J (b) 1.3 10 2 J (d) 3.4 10 3 J Solution : (d) 3 NK BT 2 For 1mole of gas N = N A & N A K B R E 3 3 RT 8.31 273 3.4 103 joule 2 2 RMS SPEED (Vrms): The square root of v 2 is known as root mean square (rms) speed. E E=N 1 mv 2 2 E 1 2 =3K T = mv N 2 2 B mv 2 = 3 K B T total mass N.m Molar mass M = Total number of particles Avogadro ′ snumber N (N A ) M ⇒m=N A M Vrms = v2 = 3 KB T NA 3K TN v2 = B A M 3 K B T NA v2 = = M 3 RT M NOTE: 1 vrms ∝ 1mN 2 1 M 2 P v rms v rms 3 V 3 V 1 2 P v rms 3 Problem 1. 𝑀 [As M = mN = Total mass of the gas] Sample Problems based on Vrms The root mean square speed of hydrogen molecules of an ideal hydrogen gas kept in a gas chamber at 0°C is 3180 m/s. The pressure on the hydrogen gas is 2 Solution : (d) Problem 2. (Density of hydrogen gas is 8 .99 10 kg / m , 1 atmosphere 1 .01 10 N / m ) (a) 0.1 atm (b) 1.5 atm (c) 2.0 atm (d) 3.0 atm 1 1 2 (8 . 99 10 2 ) (3180 ) 2 3 .03 10 5 N /m 2 3 . 0 atm As P v rms 3 3 A flask contains 10 3 3 5 2 m 3 gas. At a temperature, the number of molecules of oxygen are 3.0 10 22 . The mass of an oxygen molecule is 5.3 10 26 kg and at that temperature the rms velocity of molecules is 400 m/s. The pressure in N / m 2 of the gas in the flask is Solution : (a) (a) 8.48 10 4 (b) 2 . 87 10 4 (c) 25 .44 10 4 V 10 3 m 3 , N 3.0 10 22 , m 5.3 10 26 kg , v rms 400 m /s P Problem 3. Solution : (c) Problem 4. Solution : (a) 1 mN 2 1 5 .3 10 26 3 .0 10 22 v rms (400 ) 2 8 . 48 10 4 N /m 2 . 3 V 3 10 3 A gas at a certain volume and temperature has pressure 75 cm. If the mass of the gas is doubled at the same volume and temperature, its new pressure is (a) 37.5 cm (b) 75 cm (c) 150 cm (d) 300 cm 1 M 2 MT P v rms P 3 V V At constant volume and temperature, if the mass of the gas is doubled then pressure will become twice. At room temperature, the rms speed of the molecules of certain diatomic gas is found to be 1930 m/s. The gas is (a) H 2 (b) F2 (c) O 2 (d) Cl 2 Solution : (b) 3 RT 1930 m /s M Root means square velocity v rms M Problem 5. (d) 12 . 72 10 4 3 RT 2 (given) 3 8.31 300 2 10 3 kg 2 gm i.e. the gas is hydrogen. 1930 1930 (1930 ) The root mean square speed of hydrogen molecules at 300 K is 1930 m/s. Then the root mean square speed of oxygen molecules at 900 K will be 1930 m/s (a) 1930 3 m / s (b) 836 m/s (c) 643 m/s (d) 3 v rms 3 RT M v H2 v O2 TH 2 M H2 M O2 TO2 1930 v O2 300 32 1930 3 836 m /s . v O2 2 900 4 Graham's law of diffusion: The rate of effusion of a gas is inversely proportional to the square root of the mass of its particles. Rate of diffusion r ∝ 1 𝑀 Sample Problems based on Graham's law of diffusion Problem 1. The ratio of rms speeds of the gases in the mixture of nitrogen oxygen will be (a) 1 : 1 Solution : (c) vrms (b) v N2 3 RT v O2 M M O2 M N2 (c) 3 :1 32 28 8 7 8: 7 (d) 6: 7 APPENDIX AVOGADRO’S LAW (OR HYPOTHESIS): Equal volume of all the gases under similar conditions of temperature and pressure contain equal number of molecules. According to kinetic theory of gases PV 1 2 m N v rms 3 1 2 m 1 N 1 v rms (1) 3 1 2 For second gas, PV m 2 N 2 v rms (2) 3 …..(i) From (i) and (ii) …..(iii) For first gas, PV …..(ii) 2 2 m1 N 1 v rms 1 m 2 N 2 v rms 2 As the two gases are at the same temperature their internal energies are same 1 1 2 2 2 2 m1 vrms m2 vrms 1 2 m 1 v rms 1 m 2 v rms 2 2 2 So from equation (iii) we can say that N 1 N 2 . This is Avogadro’s law. …..(iv) For a mixture of non-reactive ideal gases, the total pressure gets contribution from each gas in the mixture 1 P = 3 [m1 n1 v 2 1 + m2 n2 v 2 2 + m3 n3 v 2 3 … … ] In equilibrium, the average kinetic energy of the molecules of different gases will be equal. That is 1 2 1 1 3 m1 v 2 1 = 2 m2 v 2 2 = 2 m3 v 2 3 … … = 2 K B T 1 ∴ P = 3 [n1 + n2 + n3 … … … . . ]3 K B T P = [n1 + n2 + n3 … … … . . ] K B T which is Dalton’s law of partial pressures. Sample problems based on Dalton's law Problem 1. The capacity of a vessel is 3 litres. It contains 6 gm oxygen, 8 gm nitrogen and 5 gm CO 2 mixture at 27°C. If R = Solution : (a) 8.31 J/mole kelvin, then the pressure in the vessel in N / m 2 will be (approx.) (a) 5 10 5 (b) 5 10 4 (c) 10 6 (d) 10 5 RT 2 RT 3 RT RT m m RT m 1 [ 1 2 3 ] 2 3 Dalton’s law P P1 P2 P3 1 V V V V V M1 M 2 M 3 8 .31 300 6 8 5 498 10 3 ~ 500 10 3 ~ 5 10 5 N /m 2 . 3 32 28 44 3 10 …TO BE CONTINUDE