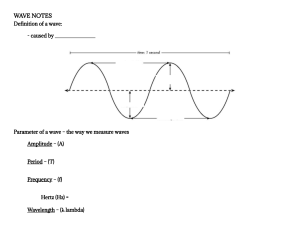
Physical Science Quarter 2 – Module 9: Dual Nature of Electrons CO_Q2_Physical Science SHS Module 9 Physical Science Alternative Delivery Mode Quarter 2 – Module 9: Dual Nature of Electrons First Edition, 2021 Republic Act 8293, section 176 states that: No copyright shall subsist in any work of the Government of the Philippines. However, prior approval of the government agency or office wherein the work is created shall be necessary for exploitation of such work for profit. Such agency or office may, among other things, impose as a condition the payment of royalties. Borrowed materials (i.e., songs, stories, poems, pictures, photos, brand names, trademarks, etc.) included in this module are owned by their respective copyright holders. Every effort has been exerted to locate and seek permission to use these materials from their respective copyright owners. The publisher and authors do not represent nor claim ownership over them. Published by the Department of Education Secretary: Leonor Magtolis Briones Undersecretary: Diosdado M. San Antonio Development Team of the Module Writers: Marilou T. Flores and Ma. Clarinda N. Medequiso Editors: Priscila D. Domino, Felipa A. Morada Reviewers: Rogelio D. Canuel, Elmer C. Bobis, Felipa A. Morada, Constancio T. Clopino, Jr. Illustrator: Alvin G. Alejandro Layout Artist: Pamela A. Lalusin, Elsie R. Reyes, Mary Grace L. Asa Management Team: Francis Cesar B. Bringas Job S. Zape, Jr. Ramonito Elumbaring Reicon C. Condes Elaine T. Balaogan Fe M. Ong-ongowan Homer N. Mendoza Catherine V. Maranan Lorna R. Medrano Edita T. Olan Editha M. Malihan Printed in the Philippines by ________________________ Department of Education – Region IV-A CALABARZON Office Address: Telefax: E-mail Address: Gate 2 Karangalan Village, Barangay San Isidro Cainta, Rizal 1800 02-8682-5773/8684-4914/8647-7487 region4a@deped.gov.ph Physical Science Quarter 2 – Module 9: Dual Nature of Electrons Introductory Message This Self-Learning Module (SLM) is prepared so that you, our dear learners, can continue your studies and learn while at home. Activities, questions, directions, exercises, and discussions are carefully stated for you to understand each lesson. Each SLM is composed of different parts. Each part shall guide you step-bystep as you discover and understand the lesson prepared for you. Pre-tests are provided to measure your prior knowledge on lessons in each SLM. This will tell you if you need to proceed on completing this module or if you need to ask your facilitator or your teacher’s assistance for better understanding of the lesson. At the end of each module, you need to answer the post-test to self-check you’re learning. Answer keys are provided for each activity and test. We trust that you will be honest in using these. In addition to the material in the main text, Notes to the Teacher are also provided to our facilitators and parents for strategies and reminders on how they can best help you on your home-based learning. Please use this module with care. Do not put unnecessary marks on any part of this SLM. Use a separate sheet of paper in answering the exercises and tests. And read the instructions carefully before performing each task. If you have any questions in using this SLM or any difficulty in answering the tasks in this module, do not hesitate to consult your teacher or facilitator. Thank you. ii What I Need to Know This module was designed and written with you in mind. It is here to help you master the use of simple collision theory to explain the effects of concentration, temperature, and particle size on the rate of reaction. The scope of this module permits it to be used in many different learning situations. The language used recognizes the diverse vocabulary level of students. The lessons are arranged to follow the standard sequence of the course. But the order in which you read them can be changed to correspond with the textbook you are now using. The module is divided into two lessons, namely: • • Lesson 1 – Is an Electron a Particle or a Wave? Lesson 2 – Evidence of Wave-like Behavior of Electrons After going through this module, you are expected to: 1. describe a particle and a wave, 2. identify key persons and their contribution in the development of the waveparticle duality theory. 3. perform an experiment to observe how electrons behave as a particle and a wave, and 4. recognize the significance of understanding the dual behavior of electrons for succeeding discoveries related to the nature of light. What I Know Directions: Choose the letter of the best answer. Write the chosen letter on a separate sheet of paper. 1. A minute portion of matter and was known as the smallest building block of the universe. A. Particle B. Photon C. Portion D. Product 2. The theory that states all matter and light shows the characteristics of both wave and particle. A. Dual wave-portion theory B. Wave-dual particle theory C. Wave-particle duality theory D. Duality of wave-product theory 1 CO_Q2_Physical Science SHS Module 9 3. A point particle with a negative electric charge. A. Atom B. Electron C. Neutron D. Proton 4. A disturbance that travels through a space-time. A. Diffraction B. Photon C. Reflection D. Wave 5. He was the first to coin the term “electron” for the electric charge quantity. A. Albert Einstein B. Christiaan Huygens C. Joseph John Thomson D. George Johnstone Stoney 6. He discovered electron particles using cathode ray tube. A. Isaac Newton B. Francesco Grimaldi C. Joseph John Thomson D. George Johnstone Stoney 7. He proposed the particle theory of light. A. Isaac Newton B. Louis de Broglie C. Max Planck D. Thomas Young 8. He proposed the wave theory of light. A. Max Planck B. Isaac Newton C. Thomas Young D. Christiaan Huygens 9. He used the double slit experiment to observe the behavior of electrons. A. Albert Einstein B. Max Planck C. Louis de Broglie D. Thomas Young 10. He hypothesized that the wave-like behavior of electrons seen in light can also be present in matter. A. Isaac Newton B. Louis de Broglie C. Thomas Young D. Christian Huygens 2 CO_Q2_Physical Science SHS Module 9 TRUE OF FALSE Directions: Write the word TRUE if the statement is correct and FALSE if otherwise. ____________ 11. Electrons have no known mass and slightly bigger than proton. ____________ 12. Wave interference can be applied to sound and light. ____________ 13. When the crest part of a wave meets another crest, sound will be produced. ____________ 14. When there is no wave interference, no sound and light is produced. ____________ 15. The dual nature of electrons paved the way for quantum physics. Lesson 1 Is an Electron a Particle or a Wave? We all know that atom is the building block of all matter in the universe. These extremely small particles are made up of a few even smaller particles. The earliest particles discovered that make up an atom are protons, neutrons and electrons. But scientists did not stop looking for the fundamental particles of matter and what “holds” them together. Recent discoveries suggest that quarks, which make up protons and neutrons, are another type of fundamental particle. Together with the leptons, quarks make up the stuff we think of as matter. Large parts of modern physics and chemistry are based on the study of energy levels of various atomic and molecular systems. Through the advancement in technology, laboratory instruments are now able to contain and observe individual electrons while telescopes can detect electron plasma by its energy emission. All these were the result of understanding the atomic and molecular behavior of the subatomic particles, specifically the electrons. This lesson will help enhance your understanding about the molecular behavior of electrons and how its discovery led to the development of the wave-particle duality theory. 3 CO_Q2_Physical Science SHS Module 9 What’s In CROSSWORD PUZZLE ACROSS 3. Electromagnetic wave visible to the naked eye 4. Positively charged particle 5. Quantized particles DOWN 1. Negatively charged particle 2. Building block of matter Notes to the Teacher 4 CO_Q2_Physical Science SHS Module 9 What’s New Tell something about the illustrations. Relate your answers on science concepts. 5 CO_Q2_Physical Science SHS Module 9 What is It Is Electron a Particle or a Wave? This question may seem a simple one but, in the scientists’, inquisitive minds, this is not so. In science, a particle is described as a minute portion of matter and is also referred to as the smallest known building blocks of the universe. This means that everything that makes up matter and universe is called particle. So how can electrons become a wave? Well, the first thing that we need to understand is to discover more about electrons. Are you ready? If yes, then let us proceed. The electron is a subatomic particle that has a negative electric charge. It has a no known structure and is believed to be a point particle. It has a mass that is approximately 1836 times less than that of the proton. The anti-particle of the electron is called the positron which is identical to electron except that it is producing a pair (or more) of gamma ray photons. The name “electron” was introduced for the electric charge quantity in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle by Joseph John Thomson in 1897 using the cathode ray tubes that enabled him to calculate the charge to mass ratio. He won a Nobel prize for his work. Then, where does the idea of electron being a wave come from? It is like having two different worlds mold into one! A sound impossible, isn’t it? Let us continue exploring by understanding what a wave is and if electrons manifest this wave-like behavior. In physics, a wave is described as a disturbance that travels through space-time and medium accompanied by transferring energy from one place to another. A medium may be a substance or material that carries the wave. The wave medium is not the wave and it does not make the wave; it merely transports the wave from its source to other locations. Remember, waves transfer energy and not matter. Thus, waves are said to be an energy transport phenomenon. 6 CO_Q2_Physical Science SHS Module 9 Consider a slinky wave as an example. Evidently, in describing these two words, waves and particles are very different. We can say that a particle is a small thing, finite object. You can hold a particle in your hand. Particles have momentum and positions. On the other hand, waves are oscillations, they are not localized. When the waves meet, crest meets crests, it is called constructive interference. When the waves cancel each other, no interaction at all, it is called destructive interference. Photo Credit: http://www.reachoutmichigan.org/funexperiments/agesubject /lessons/ bubbles.html We can apply this wave interference in sound and light. When two waves meet, sounds are produced, light is present. When there is no wave interaction, no sounds are created and only darkness. Now, how is electron become a particle and exhibit wave-like behavior at the same time? Let us go back to memory lane by tracing how it all started. 7 CO_Q2_Physical Science SHS Module 9 Lesson 2 Evidence of Wave-like Behavior of Electrons Two famous scientists in the 1600s, Christian Huygens and Isaac Newton were both working on the theories for the behavior of light. Huygens proposed a wave theory of light while Newton’s was a “corpuscular” (particle) theory of light. Newton believed that light was made up of small particles and these particles would naturally have mass too. Since light particles have mass, he deduced that a beam of light parallel to the surface of the earth would bend downward due to the pull of earth’s gravity. On the other hand, Huygens believed that light was made up of vibrating waves perpendicular to the direction of the light travels. With this concept, he was able to formulate a way to visualize wave propagation. Huygen suggested that light wave peaks form surfaces like the layers of an onion. In a vacuum or other uniform mediums, the light waves are spherical and these wave surfaces advance or spread out as they travel at the speed of light. This Huygen’s Principle explains why light shining through a pin hole or slit will spread out rather than going in a straight line. In 1803, Thomas Young studied the interference of light waves using the double-slit experiment. By shining light through a screen with two slits equally separated, the light emerging form the two slits, spread out according to Huygen’s principle. Eventually the two wave fronts will overlap with each other. His experiment firmly supported Huygen’s wave theory of light. Later in 1815, August Fresnel supported Young’s experiments with mathematical calculations. In the early nineteenth century, diffraction (slight bending) of light had been observed which firmly support the wave theory of light over Newton’s particle theory. The term diffraction was first discovered and coined by Francesco Grimaldi, an Italian natural philosopher. In 1900 Max Planck proposed the existence of a light quantum, a finite packet of energy which depends on the frequency and velocity of the radiation. The birth of quantum physics is attributed to Max Planck’s experiment on black body radiation. 8 CO_Q2_Physical Science SHS Module 9 In 1905 Albert Einstein had proposed a solution to the problem of observations made on the behavior of light having characteristics of both wave and particle theory. Using the works of Planck on emission of light to form hot bodies, Einstein suggested that light is composed of tiny particles called photons and each photon has energy. This finding came to be known as the photon theory of light which later led to the conceptualization of quantum mechanics in the twentieth century. After the wave-particle dual behavior of electron in light was accepted, another scientist took a leap by testing the hypothesis in matter. Louis de Broglie made a bold assumption and performed experiments to confirm whether the same observation can be seen in matter. In 1924, he was able to observed wave properties of the particle when beams of electrons and neutrons were directed at crystals and diffraction patterns were seen. He concluded that everything has a wavelength but the wave properties of matter are only observable for very small objects. He showed that the wave-particle duality was not merely on light but can be exhibited by both radiation and matter. Thus, the wave-particle duality theory which states that matter and light exhibit the behaviors of both waves and particles depending upon the circumstances or condition was accepted. Further studies were made by De Broglie and he found out that the probability of finding a particle at a particular location is related to the wave associated with the particle. The larger the amplitude of the wave at a particular point, the larger the probability that the electron will be found there. Similarly, the smaller amplitude the smaller the probability. This means that the larger the objects, the smaller wavelengths can be observed. But for small objects, wavelengths are more distinct as shown in the double slit experiment with electrons. Because of his profound discovery, de Broglie won a Nobel Prize. 9 CO_Q2_Physical Science SHS Module 9 What’s More Activity 1.1 Experimental Evidence of Electrons Behaving like a Wave Atoms are the building blocks of matter. This means that everything around us is made up of atoms, both for the living and non-living things. The following video links will help you enhance your understanding about the waveparticle dual nature behavior of electrons. Watch and analyze before answering the “Activity Assessment.” Part A. Nature of light: https://www.youtube.com/watch?v=J1yIApZtLos Part B. Wave-Particle Duality: https://www.youtube.com/watch?v=qCmtegdqOOA Activity 1.2 Guide Questions Directions: Answer briefly and concisely the following questions. PART A: Nature of light 1. How did Newton view about the nature of light? ___________________________________________________________________________ ___________________________________________________________________________ 2. What particular evidence shows that light is a particle? ___________________________________________________________________________ ___________________________________________________________________________ 3. What particular evidence shows that light is a wave? ______________________________________________________________________________ ______________________________________________________________________________ 4-5. Is light a particle or a wave? ______________________________________________________________________________ ______________________________________________________________________________ PART B: Wave Particle Duality 6-8. Describe how the following behave as they enter the two slits: A. Particle ___________________________________________________________________ ______________________________________________________________________________ 10 CO_Q2_Physical Science SHS Module 9 B. Wave _____________________________________________________________________ ______________________________________________________________________________ C. Quantum objects _________________________________________________________ ______________________________________________________________________________ 9. What do you think will happen if an observer modifies the experiment? ______________________________________________________________________________ ______________________________________________________________________________ 10. Briefly explain the Wave-Particle Duality theory. ______________________________________________________________________________ ______________________________________________________________________________ What I Have Learned Directions: Briefly describe the illustrations by citing scientific explanation based on the wave particle duality theory. __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ 11 CO_Q2_Physical Science SHS Module 9 __________________________________________________________________________________ __________________________________________________________________________________ What I Can Do Using the suggested materials, try to perform this experiment at home for you to have an actual observation on how electrons behave as a particle and a wave. Needed Materials: • Laser (be careful not to shine this in anyone’s eyes) • Needle • Tape • Table • White printer paper • Dark room • Flat wall Procedure: 1. Fold and unfold your sheet of printer paper once so that it can stand upright. 2. Make a tiny hole in your paper with your needle. 3. Stand your printer paper upright on a table that is at least ten feet away from the wall. 4. Use your tape to mount your laser pointer to a stable object, like a heavy book. Place the mounted laser on the table. 12 CO_Q2_Physical Science SHS Module 9 5. Turn your laser on. Adjust the angle of your laser so that it passes through the hole in your paper and onto the wall. What did you see? Is it what you expected to see? 6. Make another hole in your paper right next to the first one so that they’re as close together as possible without creating one larger hole. 7. Adjust your laser so that it now passes through both holes. Observe the shapes created on the wall. What do you see? Was it what you expected to see? 8. Cover one of the holes with a small piece of paper, leaving the other open. How does the projected image on the wall change? Guide Questions: 1. Describe the pattern of light as it passes through: A. One slit _________________________________________________________________ B. Two slits _________________________________________________________________ 2. How does the projected image on the wall change when one of the holes was covered? ______________________________________________________________________________ ______________________________________________________________________________ 3. What can you infer from the activity about the behavior of light particles? ______________________________________________________________________________ ______________________________________________________________________________ Assessment MULTIPLE CHOICE Directions: Choose the LETTER of the correct answer. 1. Light demonstrates the characteristics of______. A. Particle C. Both wave and particle B. Wave D. Neither particle nor wave 2. Wave particle duality best applies in analyzing the motion of ________. A. Projectile C. Heavenly bodies B. Space shuttle D. Electrons 3. Which phenomenon best supports the theory that matter has a wave nature? A. Electron momentum C. Photon momentum B. Electron diffraction D. Photon diffraction 4. On the atomic level energy and matter exhibit the characteristics of _______. A. Particles only C. Neither particles nor waves B. Waves only D. Both particles and waves 13 CO_Q2_Physical Science SHS Module 9 5. What does the theory of modern Physics tells us about light? A. Demonstrate wave property only B. It combines wave and particle properties C. Exclusively shows particle property D. It has neither wave nor particle properties 6. Which of the following is an example of light behaving like a particle? A. Photoelectric effect C. Interference B. Doppler effect D. Diffraction 7. What was the first experiment to show that light is a wave? A. The oil drop experiment B. The gold foil experiment C. The double-slit experiment D. The propagation of wave experiment 8. Why is laser light is used in double-slit experiment? A. It is made up of different wavelengths. B. It is made up of coordinated waves of exactly the same wavelengths. C. It is made up of uncoordinated beam of light. D. It is made up of intense beam of light. 9. What wave -like property of light is shown when light bends as enters an opening? A. Reflection C. Refraction B. Diffraction D. Interference 10. What does the dark fringe patterns of light wave on the screen in double slit experiment show? A. Destructive interference C. Constructive interference B. Reflected interference D. Diffracted interference MATCHING TYPE: Directions: Match the scientist in Column A with their contribution in Column B. Write the LETTER of the answer on the space provided. COLUMN A ______ 11. Christiaan Huygens COLUMN B A. Proposed the particle theory of light ______ 12. Isaac Newton B. Proposed light as electromagnetic wave ______ 13. Louis De Broglie C. Proved the dual nature of electron in light, radiation, and all matter ______ 14. Max Planck D. Performed the double-slit experiment ______ 15. Thomas Young E. Originator of quantum physics F. Proposed the wave theory of light 14 CO_Q2_Physical Science SHS Module 9 Additional Activities Research Work Directions: Research at least one invention made from the discovery of the waveparticle duality of electrons. Choice of presentation can be through PowerPoint or Microsoft word/WPS application. Rubric for Grading The research work will be scored from 1 to 5, with 5 being the highest in every criterion. The criteria for grading are as follows: Criteria Organization and Content Picture Cited Resources Timeliness Expectations Organization of idea and content is accurate. Language used is in own words, not copy pasted from the source. Clear and authentic. Image should bear the credit source below the picture. Follows the Chicago Manual style in citing references. Should be submitted on time. Deduction of one point per day will be administered. WORD SEARCH ACTIVITY Directions: Find and encircle the missing words hidden in the grid. The words may be hidden in any direction. Wave Particle Interference Electron Medium 15 CO_Q2_Physical Science SHS Module 9