Chemistry Summative Assessment: Thermodynamics & Electrochemistry
advertisement

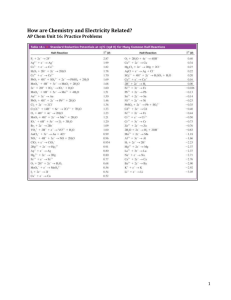
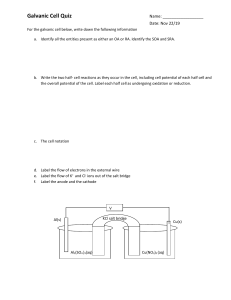
Unit 9 Summative Assessment Practice Show your work for each question in the space provided. Examples and equations may be included in your responses where appropriate. For calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Pay attention to significant figures. CO(g) + 2 H2(g) → CH3OH(g) 1. ΔH° = –90.2 kJ/molrxn Answer the following questions based on the reaction represented by the equation shown above. The values of the standard molar entropies of the substances involved in the reaction are given in the following table. Substance S° (J/(K·mol)) CO(g) 197.7 H2(g) 130.6 CH3OH(g) 239.9 (a) Use the data in the table to calculate the value of the standard entropy change, ΔS°, in units of J/(K·molrxn) for the reaction. Show your calculations in the space below. (b) Circle one of the following choices that best describes the thermodynamic favorability of this reaction. Not favored at any T Favored at all T Favored at high T Favored at low T (c) Calculate the value of the standard free energy change, ΔG°, in units of kJ/molrxn, for the reaction at 298 K. Show your calculations in the space below. (d) Is the reaction thermodynamically favorable at a temperature of 298 K? Justify your answer. 2. Chemical Equation for the Reaction ΔH° (kJ/molrxn) ΔS° (J/ K·molrxn) CO2(g) + 2 NH3(g) → CO(NH2)2(s) + H2O(l) –133.3 –424.6 Answer the following questions related to the information shown above. (a) Use particle-level reasoning to explain why the sign of the standard entropy change, ΔS°, is negative for this reaction. (b) This reaction is thermodynamically favorable at 298 K. Which of the following represents the driving force for this reaction? (Circle one of the following choices.) ΔH° only ΔS° only both ΔH° and ΔS° (c) Justify your choice in part (b) in terms of the standard free energy change, ΔG°, and the signs of ΔH° and ΔS° for this reaction. Although this reaction is thermodynamically favorable at 298 K, it is observed that the reaction occurs at a very slow rate. (d) A common explanation for the slow reaction rate is the following. The value of ( Ea ΔG° ) for this reaction has a relatively ( low high ) magnitude. When a suitable catalyst is added to the reaction mixture at 298 K, it is observed that the reaction rate increases. (e) If a suitable catalyst is added to the reaction mixture, the magnitude of the activation energy (Ea) for the reaction will ( decrease increase remain the same ). (f) If a suitable catalyst is added to the reaction mixture, the value of the standard free energy change (ΔG°) for the reaction will ( decrease increase remain the same ). N2O4(g) → 2 NO2(g) 3. Answer the following questions related to the reaction represented by the equation shown above. The value of the standard free energies of formation of the substances involved in the reaction are given in the following table. Substance G of (kJ/mol) N2O4(g) 99.8 NO2(g) 51.3 (a) Use the data in the table to calculate the value of the standard free energy change, ΔG°, in units of kJ/molrxn for the reaction. Show your calculations in the space below. (b) Is the reaction thermodynamically favorable under standard conditions? Justify your answer. (c) Calculate the value of the equilibrium constant Kp under standard conditions at a temperature of 298 K. Show your calculations in the space below. 4. Temperature Kp 300 K 0.0024 500 K 410 The value of the equilibrium constant Kp for a certain chemical reaction varies with temperature, as shown in the table above. Use the information in the table to answer the following questions about this chemical reaction. (a) Circle the correct choices for the substances that are favored at equilibrium in this reaction at temperatures of 300 K and 500 K. Assume that ΔH° and ΔS° are independent of temperature. Temperature Are Reactants or Products Favored at Equilibrium? 300 K reactants products 500 K reactants products (b) Identify the sign of ΔG° for this reaction at 300 K and at 500 K. Assume that ΔH° and ΔS° are independent of temperature. Sign of ΔG° for the Reaction at 300 K negative positive Sign of ΔG° for the Reaction at 500 K negative positive (c) Identify the signs of ΔH° and ΔS° for this reaction. Assume that ΔH° and ΔS° are independent of temperature. ΔH° negative positive ΔS° negative positive (d) Justify your choices in part (c) in terms of the Gibbs free energy equation (ΔG° = ΔH° – TΔS°) and your answer to part (b). (e) Circle one of the following choices that best describes the thermodynamic favorability of this reaction. Not favored at any T Favored at all T Favored at high T Favored at low T 5. Aluminum hydroxide, Al(OH)3(s), is produced according to the equation below. Overall Reaction: 4 Al(s) + 3 O2(g) + 6 H2O(l) → 4 Al(OH)3(s) Two half-reactions and standard reduction potentials are given in the following table. Half-Reaction Balanced Chemical Equation Al(OH)3(s) + 3 e– → Al(s) + 3 OH–(aq) 1 2 O2(g) + 2 H2O(l) + 4 e– → 4 OH–(aq) E° (V) –2.31 +0.40 (a) In the table below, write the modified versions of half-reactions 1 and 2 that will produce the overall reaction when they are added together. Modified Version of Half-Reaction 1 Modified Version of Half-Reaction 2 Overall Reaction 4 Al(s) + 3 O2(g) + 6 H2O(l) → 4 Al(OH)3(s) (b) Calculate the standard cell potential, E°, in units of volts, for the overall reaction. Show your calculations in the space below. (c) Based on your answer to part (b), calculate the value of the standard free energy change, ΔG°, in units of kJ/molrxn, for the overall reaction. Show your calculations in the space below. 5. (continued) Overall Reaction: 4 Al(s) + 3 O2(g) + 6 H2O(l) → 4 Al(OH)3(s) ΔH° = –3393 kJ/molrxn (d) The standard enthalpy change, ΔH°, for the overall reaction is –3393 kJ/molrxn. Based on this information and your answer to part (c), calculate the standard entropy change, ΔS°, in units of J/(K·molrxn), for the overall reaction at 298 K. Show your calculations in the space below. ____________________________________________________________________________________ Reaction 1 6. ΔG° (kJ/molrxn) Chemical Equation CaCO3(s) → CaO(s) + CO2(g) +131.1 2 C(s) + O2(g) → CO2(g) –394.4 3 CaCO3(s) + C(s) + O2(g) → CaO(s) + 2 CO2(g) ? Answer the following questions related to the information shown above. (a) Use the information in the table to calculate the value of the standard free energy change, ΔG°, for reaction 3 in units of kJ/molrxn. Show your calculations in the space below. (b) Reaction 1 is ( not favorable favorable ) under standard conditions. Reaction 2 is ( not favorable favorable ) under standard conditions. Reaction 3 is ( not favorable favorable ) under standard conditions. 7. Half-Reaction E° (V) Al3+(aq) + 3 e– → Al(s) –1.66 Ni2+(aq) + 2 e– → Ni(s) –0.26 A student sets up a standard galvanic cell, with an Al(s) electrode immersed in 1.0 M Al(NO3)3(aq) and a Ni(s) electrode immersed in 1.0 M Ni(NO3)2(aq). Assume that the temperature is 25°C. Two half-reactions and standard reduction potentials are given in the table above. (a) Write the balanced net ionic equation for the reaction that occurs as this galvanic cell operates. (b) Calculate the value of the standard cell potential, E°, in units of volts, for the chemical reaction that occurs as this cell begins to operate. Show your calculations in the space below. (c) Identify the anode and the cathode in this galvanic cell. anode Al(s) Ni(s) cathode (d) On the diagram above, draw an arrow to indicate the direction of electron flow in the wire as the galvanic cell operates. (e) A solution of potassium nitrate, KNO3(aq), is used in the salt bridge. The particle diagram shown at right represents an expanded view of the center portion of the salt bridge. Draw arrows in this diagram to indicate the direction of movement for the K+(aq) ions and the NO3–(aq) ions in the salt bridge as the cell begins to operate. Al(s) Ni(s) 7. (continued) (f) As this galvanic cell operates over time, will the mass of the Al(s) electrode decrease, increase, or remain the same? Justify your answer. A second galvanic cell is set up with identical conditions to the first galvanic cell, except for a change in the concentration of Al3+(aq) in the half-cell container as shown below. Galvanic Cell #1 Galvanic Cell #2 [Al3+] [Ni2+] [Al3+] [Ni2+] 1.0 M 1.0 M 0.010 M 1.0 M (g) Calculate the value of the reaction quotient, Q, for galvanic cell #2 at the moment that the cell begins to operate. Show your calculations in the space below. (h) Do you predict that the cell potential for galvanic cell #2 will be less than, greater than, or equal to the value of the cell potential calculated in part (b) for galvanic cell #1? Justify your answer. 8. An external direct-current power supply is connected to two graphite electrodes immersed in a container of molten PbBr2, as shown in the diagram above. As the cell operates, Br 2(g) is produced at one electrode and Pb(l) is formed at the other electrode. Two half-reactions and standard reduction potentials are given in the following table. Half-Reaction E° (V) Br2 + 2 e– → 2 Br– 1.07 Pb2+ + 2 e– → Pb –0.13 (a) Write a balanced net ionic equation for the electrolysis reaction that occurs in the cell. (b) Write the net ionic equation for the half-reaction that occurs at each electrode in this electrolytic cell. Half-Reaction that Occurs at the Anode Half-Reaction that Occurs at the Cathode (c) Calculate the value of the standard cell potential (E°), in units of volts, for the electrolysis reaction that occurs in this cell. Show your calculations in the space below. 8. (continued) (d) Based on your answer to part (c), calculate the value of the standard free energy change, ΔG°, in units of kJ/molrxn, for the electrolysis reaction that occurs in this cell. Show your calculations in the space below. (e) An electric current of 3.00 amperes passes through the sample of molten PbBr 2 for a period of 7.50 minutes. Calculate the mass, in grams, of Pb that is produced during this time period.