b
a
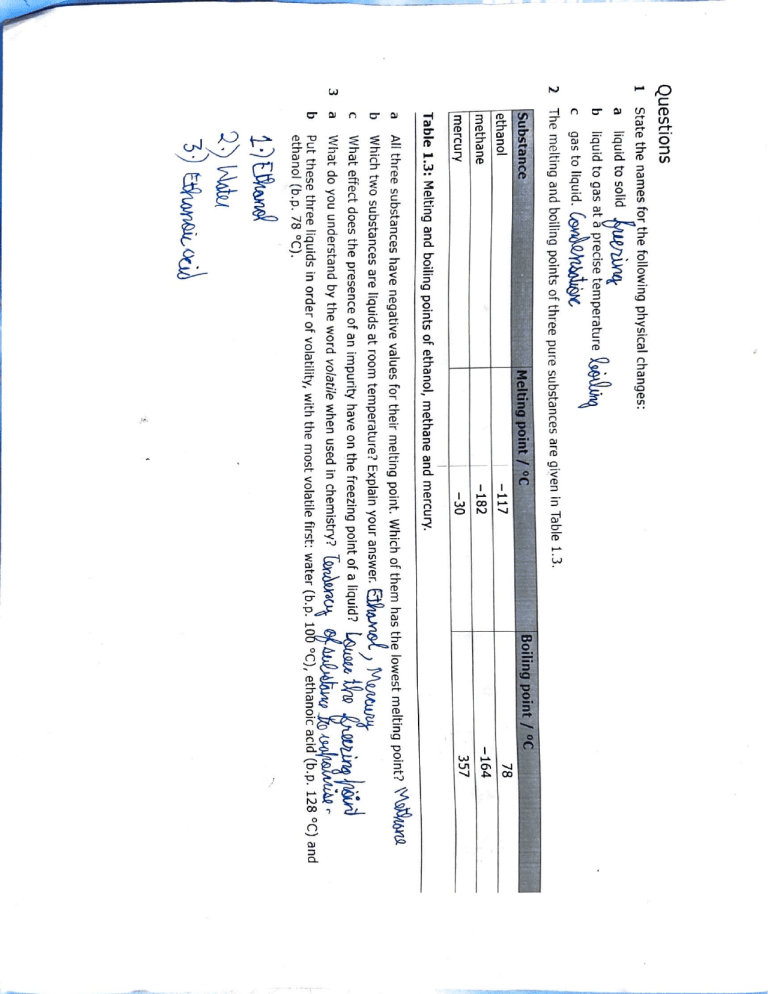
gas to liquid.
liquid to gas at a precise temperature
liquid to solid
.
ethanol
-182
-117
-164
b
a
What effect does the presence of an impurity have on t_he freezing point of a liquid?
Which two substances are liquids at room temperature? Explain your answer.~)
All three substances have negative values for their melting point. Which of them has the lowest melting point?
357
c
What do you understand by the word volatile when used in chemistry?
fJI...~ Jo~,.
-U-W
a
Put these three liquids in order of volatility, with the most volatile first: water (b.p. 10~ °C), ethanoic acid (b.p. 128 °C) and
ethanol (b.p. 78 °C).
mercury
-30
I
Table 1.3: Melting and boiling points of ethanol, methane and mercury.
methane
iSubstancc;
The melting and boiling points of three pure substances are given In Table 1.3.
c
State the names for the following physical changes:
Questions
1
2
3
b
1.~~
?)~
3)~ou1
f
0
-1
5
5
10
5
15
7
20
25
13
28
30
45
35
40
62
76
45
79
so
55
82
60
82
1 A sample of a solid substance, which had been cooled to -5 °c, was put Into a test-tube.
The test-tube was then heated In a water bath. The temperature of the substance was taken every
-5
5 minutes for an hour. The results obtained are shown below.
Time/min
Temperature/°C
•
.
'
•. I•
t·
l
I
•
'
I
'T
'
1
I
().
'
; •
r:;
++
~-
••
t t
l
l
I
•·
l
I
.,,
Ii
I
.
I
I
I
•
l
I
I
. I
•
I
r
I
JI
1
r -•
I
.
•
L+
• - ~.,
'
1 . ,
•
;-L...
1 --t-
-
I.
I -f
·-r---
T :1
- -
i.i-1 I
l
! ,- ' tP
ti
T
-l
-, '. 1
!
1
LL
:i~
'T 'J 1 :J ·r;f '
.•
j
' 1 . - f i I t l' • ' I
-1
!
,.
1
... ,f , ,, - ,....11 J'r·
.
__;_j • _;__ - ..
l
•,
'I
.I
: )· 1
' . r, , II
'-t • • f -- 7 L
J
I
·I• 1•,
l
1
r-.
·
,·- , .1 1I 1 ,f ,-t1 t ,
'•
'1
'.
·,·
-·--·.
1 1;
l;l;
Tf1 'tr~ ij'
. I . . . . i . • -!
,;f.
i
•.;
·"~
J ti.
'-0
1
-;-
_1
{4}
a Plot the results on the graph paper below, putting time on the horizontal axis and temperature
on the vertical axis.
-~
,~.,,-
...,J
0
-,o
7
T5
60
½5'
3o
[5,
...
7>
~···
r ·r:f't-· 1)f •:: <r· rc:
<-T )·f 1>, c:_,"•,r •(i:,
-is=
.j_,
{1]
')
::5
..
[1}
b What Is the melting point of the substance? ......
c What Is the boiling point of the substance? .....:!:.S'.~.....
d Describe what Is happening to the particles of ~he substance after SO minutes.~
~.