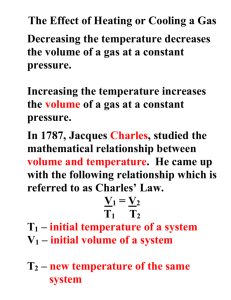
Name: Section: Date: Name: Section: Quiz #3 Boyles Law and Charles Law Part A: Read and write the letter of the correct answer. Date: Quiz #3 Boyles Law and Charles Law Part A. Read and write the letter of the correct answer. 1. Which among the units of measurement below can be the correct unit of volume? a. ° F, ° C, and K c. L, mL, m3, and cm3 b. atm, mmHg, Pa, and torr d. Kg, g, and moles 1. Which among the units of measurement below can be the correct unit of volume? a. ° F, ° C, and K c. L, mL, m3, and cm3 b. atm, mmHg, Pa, and torr d. Kg, g, and moles 2. Which of the following is a correct equation for Boyle’s Law? a. P1V1 = P2V2 c. V1/T1 = V2/T2 b. V1T1 = V2T2 d. P1/T1 = P1/T2. 2. Which of the following is a correct equation for Boyle’s Law? a. P1V1 = P2V2 c. V1/T1 = V2/T2 b. V1T1 = V2T2 d. P1/T1 = P1/T2. 3. 3. Which statement describes the volume-pressure relationship in gases at constant temperature? a. As pressure decreases, volume increases. b. As pressure increases, volume also increases. c. As pressure increases, volume remains constant. d. As pressure remains constant, volume increases Which statement describes the volume-pressure relationship in gases at constant temperature? a. As pressure decreases, volume increases. b. As pressure increases, volume also increases. c. As pressure increases, volume remains constant. d. As pressure remains constant, volume increases 4. 4. Which of the following scientists pioneered the experimentation of volume and temperature relationships at constant pressure? a. Amedeo Avogadro c. Jacques Charles b. Gay-Lussac d. Robert Boyle Which of the following scientists pioneered the experimentation of volume and temperature relationships at constant pressure? a. Amedeo Avogadro c. Jacques Charles b. Gay-Lussac d. Robert Boyle 5. 5. In order for the relationship between temperature and volume described by Charles' Law to work, what must be the unit of temperature? a. Celsius (°C) c. Kelvin (K) b. Fahrenheit (°F) d. Pascal (Pa) In order for the relationship between temperature and volume described by Charles' Law to work, what must be the unit of temperature? a. Celsius (°C) c. Kelvin (K) b. Fahrenheit (°F) d. Pascal (Pa) 6. 6. Which of the following does not involve the application of Charles’ Law? a. Baking b. Bloated tire c. Hot air balloon d. Syringe Which of the following does not involve the application of Charles’ Law? a. Baking b. Bloated tire c. Hot air balloon d. Syringe 7. 7. Why is a gas easier to compress than a liquid or a solid? a. Its volume increases more under pressure than an equivalent volume of liquid does. b. Its volume increases more under pressure than an equivalent volume of solid does. c. The space between gas particles is lesser than the space between liquid or solid particles. d. The volume of a gas’s particles is small compared to the total volume of the gas. Why is a gas easier to compress than a liquid or a solid? a. Its volume increases more under pressure than an equivalent volume of liquid does. b. Its volume increases more under pressure than an equivalent volume of solid does. c. The space between gas particles is lesser than the space between liquid or solid particles. d. The volume of a gas’s particles is small compared to the total volume of the gas. 8. 8. If the volume of a container of gas is lessened, what will happen to the pressure inside the container? a. The pressure will increase. b. The pressure will not change. c. The pressure will decrease. d. The pressure will depend on the type of gas. If the volume of a container of gas is lessened, what will happen to the pressure inside the container? a. The pressure will increase. b. The pressure will not change. c. The pressure will decrease. d. The pressure will depend on the type of gas. 9. 9. Which quantity remains constant in defining Charles’s law? a. Force b. Pressure c. Temperature d. Volume Which quantity remains constant in defining Charles’s law? a. Force b. Pressure c. Temperature d. Volume 10. What is the equivalent value of 25⁰C in Kelvin? a. 11K b. 248.15K c. 298.15K d. 6,829K Part B. Solve the following problems. Include the following details in your answer: GIVEN, FORMULA, SOLUTION, FINAL ANSWER 10. What is the equivalent value of 25⁰C in Kelvin? a. 11K b. 248.15K c. 298.15K d. 6,829K Part B. Solve the following problems. Include the following details in your answer: GIVEN, FORMULA, SOLUTION, FINAL ANSWER 1. 1. 2. A gas occupies a volume of 5.4 L at a pressure of 1.06 atm. What volume will the gas occupy when the pressure increases to 1.52 atm? Assume the temperature does not change. Oxygen gas is at a temperature of 40° C when it occupies a volume of 2.3 liters. To what temperature should it be raised to occupy a volume of 6.5 liters? 2. A gas occupies a volume of 5.4 L at a pressure of 1.06 atm. What volume will the gas occupy when the pressure increases to 1.52 atm? Assume the temperature does not change. Oxygen gas is at a temperature of 40° C when it occupies a volume of 2.3 liters. To what temperature should it be raised to occupy a volume of 6.5 liters?