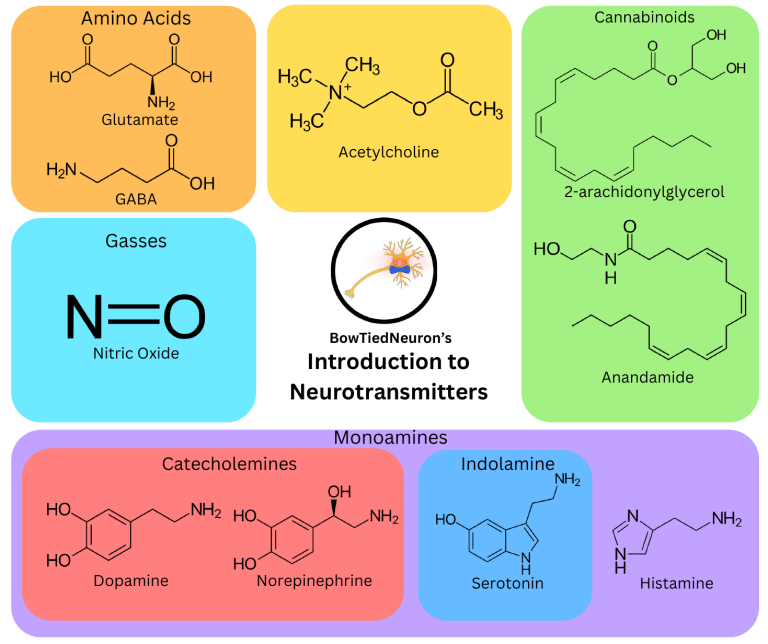
Amino Acids Cannabinoids Glutamate Acetylcholine 2-arachidonylglycerol GABA Gasses Nitric Oxide BowTiedNeuron’s Introduction to Neurotransmitters Anandamide Monoamines Catecholemines Dopamine Norepinephrine Indolamine Serotonin Histamine What is on each page: Contents: An advanced introduction to the following neurotransmitters in this order: -A brief introduction to each molecule -Glutamate -How it is synthesized -GABA -Dopamine -Norepinephrine -Serotonin -Histamine -Acetylcholine -Cannabinoids -Nitric Oxide -Their molecular structure -How it is removed from the synapse -The major receptor types and what they do -That molecules function in the brain -Location in the brain (if applicable) -A brief conclusion What you need to know: Adenylyl cyclase is an enzyme that converts ATP into cAMP, a key signaling molecule in neurons. cAMP can go on to activate enzymes, modulate receptors and more. PLC is another enzyme that starts a signaling cascade activating PKC and CaMK. 5 Major Receptor Types: AMPA: excitatory ionotropic receptor, when activated allows sodium (Na+) into the neuron making it more positive and more likely to fire an action potential Glutamate Glutamate is the primary excitatory neurotransmitter in the brain. Synthesis Glutamate is an amino acid synthesized from other amino acids, like glutamine and aspartate, or intermediates from the citric acid cycle like αketoglutarate. 1 Method of Removal from Synapse Reuptake by a glutamate transporter, Excitatory Amino Acid Transporter (EAAT), on the presynaptic neuron or nearby astrocyte NMDA: highly unique excitatory ionotropic receptor, requires binding of co-agonist (serine or glycine), removal of a magnesium ion block, and glutamate binding to open; allows both Na+ and Ca2+ into the neuron making it more positive and more likely to fire an action potential, Ca2+ also functions as a signaling molecule inside neurons making this more than your typical ionotropic receptor Kainate: excitatory ionotropic receptor; also involved in inhibiting neurotransmission (especially GABA) when located on presynaptic neurons Group 1 mGluR: stimulatory metabotropic receptor, upregulates phospholipase C, increases excitability of postsynaptic neuron and NMDA receptor activity Group 2/3 mGluR: inhibitory metabotropic receptors, inhibits adenylyl cyclase, generally a presynaptic inhibitory autoreceptor that inhibits glutamate release Conclusion Glutamate’s function is simple. It acts as the primary excitatory neurotransmitter in the brain, driving action potentials in other neurons. Things get complex when you begin to look at the function of the NMDA receptor. It is heavily implicated in plasticity at those synapses since it allows calcium into neurons, and as a result plays a large role in learning and memory. Though excess glutamate activity is detrimental and the excess calcium influx that follows can cause excitotoxicity and cell death. Just another reason to take more magnesium, sufficiently block your NMDA channels. 2 Major Receptor Types: GABA GABA is the primary inhibitory neurotransmitter in the brain. Synthesis GABA is an amino acid synthesized from glutamate via the enzyme glutamic acid decarboxylase (GAD) 2 Methods of Removal from Synapse Reuptake by a GABA transporter on the presynaptic neuron or nearby astrocyte GABA Transmaminase, the enzyme that breaks it down GABAA: inhibitory ionotropic receptor; the main source of ‘fast’ inhibitory neurotransmission in the brain, allows chloride (Cl-) into the neuron making it more negative and less likely to fire an action potential There is a huge diversity of GABAA subtypes localized to different areas in the brain with different subunit compositions. This affects binding affinity for GABA and drugs that act on these receptors (i.e. benzodiazepines, barbiturates). GABAB: inhibitory metabotropic receptor, inhibits activity of adenylyl cyclase, activate inhibitory potassium (K+) channels, can function as inhibitory autoreceptor on the presynaptic neuron decreasing neurotransmitter release Conclusion GABA is a simple molecule. As the primary inhibitory neurotransmitter in the brain its chief job is to decrease excitability of neurons, reducing their chance of firing an action potential. It does so directly through the GABAA receptor or indirectly through the GABAB receptor. In general, an increase in GABA activity will decrease overall neuronal activity, and vice versa. 2 Major Receptor Types: D1: stimulatory, upregulates adenylyl cyclase Dopamine Dopamine is a catecholamine, a subtype of monoamine, along with norepinephrine and epinephrine. Synthesis Catecholamines all come from the same synthesis pathway L-Tyrosine → L-Dopa → Dopamine → Norepinephrine → Epinephrine 2 Methods of Removal from Synapse Reuptake into the presynaptic neuron by a Dopamine transporter, DAT Monoamine oxidase, the enzyme that breaks it down D2: inhibitory, downregulates adenylyl cyclase, activates inhibitory ion channels in postsynaptic cell; generally a presynaptic autoreceptor, inhibitng dopamine release 3 Major Pathways Nigrostraital (Substantia nigra →Striatum): This pathway contains most of the dopamine neurons in the brain and is involved in motor planning and movement. The striatum is part of the basal ganglia, the region of the brain responsible for initiating and stopping movement. Mesolimbic (VTA→Limbic system): This pathway is commonly referred to as the reward pathway and contains projections from the VTA to the nucleus accumbens (NAc), the primary reward synapse in the brain. The NAc is very important for motivation and taking action on it, primarily for natural rewards. This is the 'stereotypical dopamine' pathway, and is the same one that's responsible for addiction. Mesocortical(VTA→ Cortex): This pathway projects to the cortex, most importantly the frontal cortex. It has a large variety of functions including modulating attention, executive function, working memory and more. Conclusion Dopamine and its pathways are crucial for coordinating a response to specific stimuli, typically related to reward of some kind. This includes placing value on rewards and cues that predict them, influencing learning and memory, and turning goals into motor outputs. 3 Major Receptor Types: α1: stimulatory metabotropic receptor, upregulates phospholipase C (PLC) Norepinephrine Norepinephrine is a catecholamine, a subtype of monoamine, along with dopamine and epinephrine. Synthesis Catecholamines all come from the same synthesis pathway L-Tyrosine → L-Dopa → Dopamine → Norepinephrine → Epinephrine 2 Methods of Removal from Synapse Reuptake into the presynaptic neuron by a Norepinephrine transporter, NET Monoamine oxidase, the enzyme that breaks it down α2: inhibitory metabotropic receptor, inhibits adenylyl cyclase, generally acts as an autoreceptor inhibiting NE synthesis and release β1/2/3: stimulatory metabotropic receptor, upregulates adenylyl cyclase 1 Major Pathway Almost all norepinephrine neurons are located in the locus ceruleus, a part of the pons. Locus Ceruleus → Cortex, Amygdala, Hippocampus, Hypothalamus, Thalamus As a result, norepinephrine is involved in arousal, attention, threat response, promoting wakefulness, and memory Conclusion Norepinephrine is a molecule responsible for the ‘fight or flight’ response in the brain. It and epinephrine do this in the periphery as well working in the autonomic nervous system Its receptors are all metabotropic, likely working to increase excitability of the postsynaptic neuron so they can fire easier. Its main role is to promote arousal, and if you’ve ever taken an α2 antagonist like yohimbine you’ll agree (remember α2 receptors are generally autoreceptors, inhibiting an inhibitor will increase NE release). 4 Major Receptor Types: 5-HT 4/6/7: stimulatory metabotropic receptors, upregulate adenylyl cyclase Serotonin Serotonin or 5-Hydroxytryptamine (5-HT) is a monoamine, more specifically an indolamine. 5-HT 1A-1F/5: inhibitory metabotropic receptors, downregulate adenyylyl cyclase or open inhibitory ion channels, 5-HT1B/D are presynaptic autoreceptors and inhibit serotonin synthesis and release 5-HT 2A/B/C: stimulatory metabotropic receptor, upregulates PLC and subsequently PKC, and CaMK 5-HT 3: excitatory ionotropic receptor, ligand gated ion channel that allows Na+ and Ca2+ through exciting the neuron Synthesis Serotonin is synthesized from the amino acid tryptophan, and is also a precursor to melotonin. 2 Methods of Removal from Synapse Reuptake into the presynaptic neuron by a Serotonin transporter, SERT Monoamine oxidase, the enzyme that breaks it down 2 Major Pathway Dorsal Raphe → Cortex, amygdala, midbrain Median Raphe → Hippocampus, hypothalamus, thalamus As a result, serotonin plays a role in sleep, attention, mood/emotion, social behavior (and aggression, risk taking behavior), sensory processing (psychedelics are 5-HT agonists), arousal, learning and memory, and more. Conclusion Serotonin is an interesting neurotransmitter with a wide range of functionality, and we are still learning more about it. It is most well known for its effects on mood, given the use of SSRIs in treatment of depression and anxiety disorders, but it is crucial for proper functioning of other behaviors as well. 4 Major Receptor Types: H1: stimulatory metabotropic receptor, activates phospholipase C starting a signaling cascade in the cell, one of primary actions is to increase excitability of the neuron by closing potassium channels Histamine Histamine is a monoamine like dopamine and serotonin, but does not fall under a more specific group like catecholamine or indolamine. Synthesis Histamine is synthesized from the amino acid histidine. H2: stimulatory metabotropic receptor, activates adenylyl cyclase, one of primary actions is to increase excitability of the neuron by closing potassium channels, also control release of stomach acid H3: inhibitory metabotropic receptor, typically autoreceptors that inhibit histamine release H4: plays a role in inflammation outside of the nervous system, not really relevant in the brain 1 Major Pathway All histamine neurons are located in the tuberomammillary nucleus (TMN) of the hypothalamus. They project all over the brain to the striatum, cortex, hippocampus, and parts of the hypothalamus. As a result histamine is involved in, sleep/wake cycles, arousal, feeding, learning, and memory 1 Method of Removal from Synapse Histamine nmethyltransferase, the enzyme that breaks it down Conclusion Histamine is a neurotransmitter with a smaller range of functions than others. Despite this, its function is extremely influential on our daily lives through its effects on the sleep/wake cycle. Its functions are not exclusive to the brain though as it's an important signaling molecule involved in inflammation in the periphery, and even causes secretion of stomach acid. 4 Major Receptor Types: Nicotinic α1 and β1: Expressed at the neuromuscular junction Nicotinic α2-9 and β1: Expressed throughout the brain Acetylcholine Acetylcholine is a unique moleucle that functions as a neurotransmitter in the brain and at muscles, causing muscle contraction Synthesis Acetylcholine is synthesized from the precursors choline and acetyl coenzyme A. 1 Method of Removal from Synapse Acetylcholinesterase, the enzyme that breaks it down There is no acetylcholine transporter, only for choline All nicotinic receptors are excitatory ionotropic receptors, permeable to sodium and calcium. They’re composed of 5 subunits, generally that are different based on location in the brain. This affects the affinity of drugs, nicotine is not an agonist at muscle for example. Muscarinic M1,M3,M5: stimulatory metabotropic receptors, upregulates phospholipase C Muscarinic M2, M4: inhibitory metabotropic receptors, downregulates adenylyl cyclase or opens inhibitory ion channels, M2 generally acts as an autoreceptor inhibiting acetylcholine release 2 Major Pathways Basal forebrain → Cortex, limbic system, hippocampus Brainstem nuclei → VTA, thalamus, other areas in brainstem As a result, acetylcholine plays a role in learning and memory, arousal, emotional states, sensory processing, reward, sleep/wake, and movement (both in striatum and at muscles) Conclusion Acetylcholine is a fascinating neurotransmitter with a wide range of functions not only in the brain, but also at the neuromuscular junction as the neurotransmitter responsible for initiating muscle contraction. Its effects outside of the NMJ are mostly known for its positive influence on learning and memory, making it a useful target fo nootropics (i.e. nicotine, huperzine-a), but it also plays a very important role in the sleep wake cycle Cannabinoids 2 Major Receptor Types: CB1: inhibitory metabotropic receptor, located on presynatpic terminals, inhibits neurotransmitter release by inhibiting calcium channels and/or opening inhibitory potassium channels, source of psychoactive effects of THC 2-arachidonylglycerol CB2: low levels in CNS, mostly in periphery involved in inflammation and immune system How They Work Cannabinoids function as retrograde messengers, meaning they are synthesized in the postsynaptic neuron and travel to the presynaptic neuron to elicit their effect. Once synthesized they are immediately released, and they're not stored in vesicles. Anandamide Synthesis Cannabinoid synthesis is caused by activation of specific metabotropic receptors on the post synaptic neuron or a strong influx of calcium. Their action on neurons is inhibitory, specifically inhibiting neurotransmitter release. In this way, it functions as part of a negative feedback loop. Strong signal from the presynaptic neuron causes its synthesis, it travels back and inhibits release through its action on CB1. Conclusion This is a very complex neurotransmitter system and more is still being learned about it to this day, but this should be a They're synthesiezd from lipid good introduction to understanding what they are and how precursors in the postsynaptic they work within the brain. The important takeaway being that neuron. Different cannabinoid they function in a negative feedback loop to decrease molecules have different synthesis neurotransmitter release. . pathways How It Works . Nitric Oxide Nitric Oxide functions as a retrograde messenger, although it doesn’t have receptors like cannabinoids. Synthesis When it is synthesized in the postsynaptic neuron, it immediately travels to the presynaptic neuron where it upregulates guanylyl cyclase, an enzyme that produces cyclic GMP (cGMP). Nitric Oxide is a gaseous neurotransmitter that is very unique in its mechanism of action Nitric Oxide is synthesized in response to glutamate NMDA receptor activation. They allow calcium into the postsynaptic neuron. It binds to calmodulin, activating neuronal nitric oxide synthase (nNOS). nNOS then converts the amino acid arginine into nitric oxide. The effect this has is that it increases neurotransmitter release. It is part of a positive feedback loop, where strong glutamate signaling triggers its synthesis, then it goes on to stimulate more glutamate release. This feedback loop helps induce long-term potentiation (LTP), a form of plasticity that increases the strength of synapses. The increase in glutamate signaling it causes increases . calcium influx which will have other effects, like altering gene expression and inserting more receptors into the membrane. This physical change in the synapse plays a large part in learning and memory


