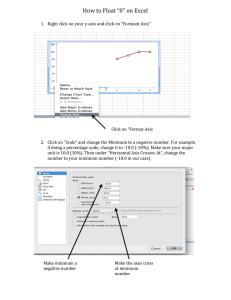
Point Groups Point Group = the set of symmetry operations for a molecule Group Theory = mathematical treatment of the properties of the group which can be used to find properties of the molecule Assigning the Point Group of a Molecule 1. Determine if the molecule is of high or low symmetry by inspection A. Low Symmetry Groups B. High Symmetry Groups 2. If not, find the principle axis 3. If there are C2 axes perpendicular to Cn the molecule is in D If not, the molecule will be in C or S 4. If h perpendicular to Cn then Dnh or Cnh If not, go to the next step 5. If contains Cn then Cnv or Dnd If not, Dn or Cn or S2n 6. If S2n along Cn then S2n 7. If not Cn The determination of point groups of molecules only one rotational two σv but no σh mirror planes means axis = C2 point group is C 2v The point group of the water molecule is C2v Naming point groups: The name of the point group has information about the symmetry elements present. The letter is the rotational group and the subscript number after the letter indicates the order of the principal rotational axis (e.g. 3-fold or 4 fold etc.): A ‘C’ indicates only one rotational axis C3 C3v 3-fold rotational has σv but axis no σh mirror planes in a C group A ‘D’ indicates an n-fold principal rotation axis plus n 2-fold axes at right angles to it D4d 4-fold principal axis D4h d = no σh mirror plane ‘h’ indicates a σh mirror plane Naming point groups (contd.): A subscript ‘h’ means that there is a σh mirror plane at right angles to the n-fold principal axis: C4 principal axis C3 principal axis only one of the three σv planes is shown σh D4h σv D3d A subscript ‘d’ (or v for C groups) means there is no σh mirror plane, but only n σv mirror planes containing the principal Cn axis. Naming platonic solids: Platonic solids: Five special polyhedra: Tetrahedron, Cube, Octahedron, Dodecahedron, and Icosahedron Their faces are all exactly the same. The same number of faces meet at each vertex. T = tetrahedral = 4 three-fold axes O = octahedral = 3 four-fold axes I = icosahedral = 6 five-fold axes C60 ‘bucky-ball’ or ‘Fullerene’ Td Oh Ih Flow chart for determining point groups. The point group of the carbon dioxide molecule i We start at the top of the C∞ D∞h flow-chart, and can see that the CO2 molecule is linear, and has a center of inversion (i) so it is D∞h. Note the C∞ principal rotation axis. Other linear molecules: The top row of linear molecules all have a center of inversion (i) and so are D∞h. D∞h i N2 O2 HC≡N The bottom row have no i and so are C∞v i F2 HI C∞v H2 C≡O All have a C∞ axis The Platonic solids: tetrahedron Td octahedron Oh icosahedron Ih C60 ‘buckyball’ The Cs point group: σ I Cs C F F Cl chloro-difluoro-iodomethane Most land animals have bilateral symmetry, and belong to the Cs point group: Cs Mirror planes (σ) The C1 point group: Molecules that have no symmetry elements at all except the trivial one where they are rotated through 360º and remain unchanged, belong to the C1 point group. In other words, they have an axis of 360º/360º = 1-fold, so have a C1 axis. Examples are: I I N Cl Br C1 C H Cl F Bromo-chloro-fluoro-iodomethane C1 chloro-iodo-amine The division into Cn and Dn point groups: After we have decided that there is a principal rotation axis, we come to the red box. If there are n C2 axes at right angles to the principal axis, we have a Dn point group, If not, it is a Cn point group. Dn Cn The Cn point groups: The Cn point groups all have only a single rotational axis, which can theoretically be very high e.g. C5 in the complex [IF6O]- below. They are further divided into Cn, Cnv, and Cnh point C5 groups. The Cn point groups have no other O symmetry elements, iodine the Cnv point groups have also n mirror planes F containing the Cn rotational axis, while the Cnh point groups also have a σh mirror F plane at right angles to the [IF6O]- principal rotational axis. The point group of the water molecule We start at the top of the flow-chart, and can see that the water molecule is not linear, and is not tetrahedral (Td), octahedral (Oh), or icosahedral, (Ih) so we proceed down the chart C2 Yes, there is a principal Cn axis, so we proceed down the chart, but in answer to the next question, there are no further C2 axes at right angles to the principal axis, which is the only axis, so we proceed down the chart C2 C2 C2 σv σv there is no σh plane at right angles to the C2 axis, but there are two σv planes containing the C2 axis. The point group of the water molecule is C2v Other Cnv molecules: water ammonia σv C2v C3v C4v V σv Vanadyl tetrafluoride (VOF4) σv Some more C2v molecules: C2 σv P C2 σv S σv Phosphorus iodotetrafluoride (PF4I) σv C σv sulfur tetrafluoride (SF4) C2 σv carbonyl chloride (COCl2) The Cn point groups: These have a Cn axis as their only symmetry element. Important examples are (hydrogens omitted for clarity): C3 triphenyl phosphine viewed down C3 axis C3 C3 C3 C3 triphenyl phosphine viewed from the side Cobalt(III) tris-glycinate viewed down C3 axis C3 Cobalt(III) tris-glycinate viewed from the side The Dnh point groups: C4 principal axis four C2 axes at rt. angles to C4 axis C2 C2 C2 mirror plane at rt. angles to C4 axis σh C2 D4h Examples of molecules belonging to Dnh point groups: C2 C4 C3 C3 D2h D3h D3h C5 C4 D4h C3 D4h D3h C5 D5h D5h Benzene, an example of the D6h point group: C6 principal axis C6 C2 σh C2 C2 C2 σv σv D6h C6 principal axis C6 principal axis The Dn point groups: these have a principal n-fold axis, and n 2-fold axes at right angles to it, but no mirror planes. C2 principal axis N C2 N C2 C [Cu(en)2]2+ complex with H-atoms omitted for clarity. (en = ethylene diamine) Cu D2 Some further views of the symmetry elements of [Cu(en)2]2+, point group D2 : C2 principal axis D2 C2 principal axis [Cu(en)2]2+ complex with H-atoms omitted for clarity. (en = ethylene diamine) C2 C2 C2 C2 C2 principal C2 principal axis axis C2 C2 C2 C2 Some views of the symmetry elements of [Co(en)3]3+, point group D3. C2 C3 principal axis C2 C2 D3 C3 principal axis view down the C3 axis of [Co(en)3]3+ showing the three C2 axes. C2 axis view down one of the three C2 axes of [Co(en)3]3+ at right angles to C3 Other examples of the D3 point group C2 C3 C2 C2 principal axis C2 C2 C2 D3 D3 [Co(oxalate)3]3- [Co(bipyridyl)3]3+ Molecules belonging to the Dnd point groups These have mirror planes parallel to the principal axis, but not at right angles to it. C5 axis C3 axis σv planes contain the principal axis D3d Staggered form of ethane D5d Staggered form of ferrocene The D4d point group: C2 C4 principal axis C2 C2 σv σv σv C2 σv C2 C4 principal axis ]4- [ZrF8 Square antiprism C4 principal axis D4d As predicted by VSEPR, the [ZrF8]4- anion has a square anti-prismatic structure. At left is seen the C4 principal axis. It has four C2 axes at right angles to it, so it has D4 symmetry. One C2 axis is shown side-on (center). There are four σv mirror planes (right), but no mirror plane at right angles to C4, so the point group does not rate an h, and is D4d. [K(18-crown-6)]+, an example of a D3d point group: C3 C2 C2 principal axis C3 principal axis K+ σv σv C2 C2 C2 C2 σv D3d The complex cation [K(18-crown-6)]+ above is an important structure that has D3d symmetry. It has a C3 principal axis with 3 C2 axes at right angles to it, as well as three σv mirror planes that contain the C3 axis, but no σh mirror plane (because it’s not flat, as seen at center), so is D3d. Some Point groups