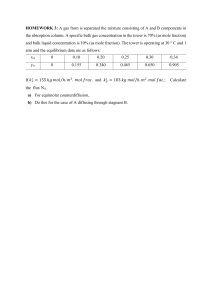
Chapter 3 Process and Process Variables Process is any operation or a series of operations which cause physical or chemical changes in a substance or a mixture of substances. input (feed) Process Unit output (product) Operating variables represent the amounts (mass flow rates), composition, and states of substances that go in and come out of process units. (ex) mass, volume, density, flow rate, mole fraction, composition, pressure, temperature, etc. In this chapter, definitions and units of the operating variables are explained. Process Operating Variables Equations Components Equipments Energy requirement (Units) (q+W) Flow rates Streams Energy balance equations Mass balance equations Compositions Temperature Pressure Energy balance equations 3.1 MASS AND VOLUME ① Density ( ) of a substance is the mass per unit volume of the substance: ② Specific volume ( ) of a substance is the volume per unit mass of the substance: Specific volume is the inverse of density. (Example) The density of The mass of . is ③ Specific gravity of a substance is the ratio of the density of the substance ( ) to the density of a reference substance ( specific condition. The reference substance most commonly used for solids and liquids is water at . ) at a The notion of to water at The SG of a substance at is with reference (See Table B.1) (Example 3.1-1) Mass, Volume and Density (a) Calculate the density of mercury in (b) Calculate the volume in . occupied by of mercury. [Solution] From Table B.1, for mercury (a) (b) 3.2 FLOW RATE 3.2a Mass and Volumetric Flow Rate Flow rate of a material is the rate at which the material is transported through a process line. Mass flow rate is in mass per time: m (kg/s) Volume flow rate in volume per time: V (m3/s) pipe fluid Cross section perpendicular to the direction of flow m/V = m / V Test Yourself on p46 1 2 (a) How do the mass flow rates of the gas at the inlet and outlet compare? (At steady state only) From the conservation of mass : m1 = m2 (b) If the density of the gas is constant, how do the volumetric flow rates at these two points compare? m = uS, V = uS if , then uS = uS, V = V (c) What if the density decreases from inlet to outlet? uS = uS --> uS = uS V = V , V < V 3.2b Flow Rate Measurement Rotameter Orifice Meter 3.3 CHEMICAL COMPOSITION 3.3a Moles and Molecular Weight The atomic weight of an element is the mass of an atom on a scale that assigns a mass of exact 12. The molecular weight of a compound is the sum of the atomic weights of the atoms that constitute the compounds. (ex) Example 3.3-1 Conversion between Mass and Moles How many of each of the following are contained in Carbon dioxide The number of molecules in mass flow rate (Avogadro number) molar flow rate of CO2 ? molar flow rate mass flow rate 3.3b Mass and Mole Fractions and Average Molecular Weight The following terms may be used to define the composition of a mixture of a substance. (1) Mass fraction: mostly used for liquid and solid mixtures , percent (%) (2) Mole fraction: mostly used for gas mixtures , percent (%) (3) Average molecular weight or mean molecular weight : Air : 79 % N2 and 21% O2 Ave MW of Air? = 0.79 x 28 + 0.21 x 32 = 28.84 = 29 ① With mole fractions and the molecular weight known: Basis: 1 mole mixture : mole fraction of component in the mixture : molecular weight of component : number of components in the mixture [Proof] Basis of calculation Total mass of 1 mol: ② With mass fractions and molecular weight known : mass fraction of component in the mixture : molecular weight of component : number of component in the mixture [Proof] Basis of calculation: (Example 3.3-3) Conversion from a Composition by Mass to a Molar Composition Basis : , 1000 kg, ... Set all the calculations in tabular form (Example 3.3-4) Calculation of an Average Molecular Weight Calculate the average molecular weight of air (1) from its molar composition: 79% N2 and 21% O2 (2) from its mass composition: 76.7% N2 and 23.3% O2. (Solution) (1) Basis: 100 mol air Component Molecular Weight Moles Mass(g) N2 28 79 79*28 O2 32 21 21*32 100 2880 Total Average molecular weight = 28.8 g/g mol (2) Basis: 1000 g air Component Molecular Weight Mass (g) Moles N2 28 767 767/28 O2 32 233 233/32 1,000 35 Total Average molecular weight = 1000 g/35 mole = 28.6 3.3c Concentrations (1) Mass concentration: Mass/volume Mass of a component per unit volume of the mixture (2) Molar concentration: Moles/volume The number of moles of a component per unit volume of the mixture (kmol/cm3, lb-mol/ft3, …) (3) Molarity (M): Moles solute/L solution (4) Molality (m): Moles solute/kg solvent . Why use molality ? Normality (N): for acids, bases, ionic compounds, Molarity of proton (H+ ) or electrons in a solution. HCl: 0.5 M HCl= 0.5 N HCl H2SO4: 0.5 M H2SO4 = 0.5*2 N H2SO4 (Example) 0.02 M NaOH If a stream of this solution flows at a rate of , (Example) Assume 10 wt % NaCl in Water ( 1 kg/L) Molarity of NaCl? = moles NaCl/L solution = 1.71 mol NaCl/L solution Molality of NaCl? = moles of NaCl/1kg solvent (H2O) = 1.71/0.9 = <Solution> (1) Basis: 1000 g Mixture of NaCl and H2O Components Molecular wt Mass Moles NaCl 58.45 100 g 100/58.45 = 1.71 H2 O 18 900 g 900/18 = 50 M NaCl = 1.71/1 L = 1.71 M m NaCl = 1.71/ 0.9 kg H2O = 1.9 m NaCl 3.3d Parts per Million (ppm) and Parts per Billion (ppb) for Trace Species 1 ppm = 1 particle (molecule) among 1 millions of total particles (molecules) 1 ppb = 1 particle (molecule) among 1 billions of total particles (molecules) (1) Liquid mixtures: mass ratio ppm = x (mass fraction) x 106, ppb = x (mass fraction) x 109 (Ex) 125 ppb phenol in water Mass fraction of phenol ?, mg phenol/kg liquid ?, mg phenol/L liquid ? Basis: 1000 g mixture Component MW Mass mol ---------------------------------------------------------------------------------------------Phenol (TB.1) 94.11 0.125 mg 0.0013 mmol H2 O 18 1000 g 55.6 mol 1 ppm = 1 mg/kg solution Exact concentration of phenol in ppb from definition = 1.3x10-6x109/(1.3x10-6+55.6) = 23.3 ppb <Dilute solution in liquids>: 1 ppm = 1 mg/L 10 ppm phenol = 10 mg phenol/1 L water (2) Gas mixtures: mole ratio ppm = y (mole fraction) x 106, ppb = y (mole fraction) x 109 (Ex) 15 ppm SO2 in air Mole fraction of SO2 = 15 x 10-6 mg SO2/L air? 3.4 PRESSURE 3.4a Fluid Pressure and Hydrostatic Head Pressure is the ratio of a force to the area on which the force acts: The fluid pressure (Figure 3.4.1) for a fluid contained in a closed vessel or flowing through a pipe. F may be defined as the ratio of F/A, where F is the minimum force that would have to be exerted on a frictionless plug in the hole to keep the fluid from emerging. Hydrostatic pressure is defined as the force exerted on the base of the base area A. (Figure 3.4.2) F equals the force on the top surface plus the weight of the fluid in the column. Head of a particular fluid; The height of a hypothetical column of the particular fluid that would exert the given pressure at its base if the pressure at the top were zero. (Ex) 1 atm ( ) (Example 3.4-1) Calculation of a Pressure as a Head of Fluid Express a pressure of 2.00 x 105 Pa in terms of mm Hg. 200,000 Pa * 1 atm/100,000 Pa * 760 mm Hg/1 atm = 1520 mm Hg (Example 3.4-2) Pressure below the Surface of a Fluid [Solution] (1) Pressure in Pa (2) Pressure in Head 3.4b Atmospheric Pressure, Absolute Pressure, and Gauge Pressure Atmospheric Pressure (Patm ) is the pressure of the atmosphere at the base of a column of air located at the point of measurement. Density of air acceleration of gravity The standard pressure of 1 atmosphere (760 mmHg) is the atmospheric pressure at sea level. Absolute pressure (Pabs) A zero pressure corresponds to a perfect vacuum . Gauge pressure (Pgauge ) is measured by many pressure-measuring devices A zero gauge pressure corresponds to an atmospheric pressure. Negative gauge pressure: Absolute pressure is lower than atmospheric pressure. -76 cm Hg - absolute pressure = 76 -76 = 0 (complete vacuum), P = 0 - 10 cm Hg P = 76 -10 = 66 cm Hg 3.4c Fluid Pressure Measurement ◎ Two types of common pressure gauges Bourdon Gauge (Figure 3.4-3) utilizes straightening of a hollow tube closed at one end and open at the other end which is exposed to the fluid. Pressure range : from vacuum to 7000 atm. Manometer is a U-shaped tube partially filled with a fluid of known Density (Figure 3.4-4). Pressure difference causes the liquid level difference. Open-end manometer measures gauge pressure Differential manometer measures pressure difference between the two points. Sealed-end manometer measures absolute pressure. Equations for manometers (1) General manometer equation (3.4-5) (2) Differential manometer equation (3) If either 1 or 2 is a gas, (Example 3.4-3) Pressure Measurement with Manometers 1. Differential Manometer in a process line containing water. = (f -w ) g h = 40 dynes/cm2 2. Open-End Mercury Manometer 3.5 TEMPERATURE (1) The temperature of a substance is a measure of the average kinetic energy possessed by the substance molecules. (2) Temperature is determined indirectly by measuring some physical property of the substance whose value depends on temperature. Resistance thermometer – electrical resistance of a conductor. Thermocouple – voltage at the junction of two dissimilar metals. Thermocouple electricity generation https://www.youtube.com/watch?v=xaT2hqHgLdY Pyrometer – spectra of emitted radiation. Bulb thermometer – Volume of a fixed mass of fluid. (3) Temperature scales Celsius (centigrade) – freezing point of water ( point ( ) Absolute zero is Fahrenheit – For H2O (l), is 32F and ) is , and the boiling at 1 atm. . is 212F. Kelvin – absolute zero is 0. The size of one degree is the same as a Celsius degree. Rankine – absolute zero is 0. The size of one degree is the same as a Fahrenheit degree. (Source: David M. Himmelblau, "Basic Principles and Calculations in Chemical Engineering", 6th ed., Prentice Hall, 1996) Relations between temperature scales * Temperature differences in various units in the numerators (3.5-1) (3.5-2) (3.5-3) (3.5-4) (ex) T difference of 100℃ corresponds to T difference of 180℉. T difference of 100℃ corresponds to T difference of 100K. T difference of 100K corresponds to T difference of 180R. Relations between temperature intervals ( ) in denominators. Conversion factors for temperature intervals: <Property changes over unit temperature difference> From Figure, Pc (J/oC) = 1.8 PF (J/oF) or Pc (J/oC) = PF (J/oF) x 1.8 oF/oC = 1.8 PF (J/oF) (Example 3.5-2) Temperature Conversion Consider the interval from to . 1. 2. (Example 3.5-3) Temperature Conversion and Dimensional Homogeneity The heat capacity of ammonia is Determine the expression for Cp in in terms of [Solution] 1. Substitute T (℃) for using equation (3.5-4). . 2. Convert the desired temperature interval using Equation (3.5-5). Using Basis for Engineering Calculation versus Using Equations for Mathematical Calculation Find the composition of air by mass fraction given that mole fractions of O2 and N2 are 0.21 and 0.79, respectively. Molecular weights of O2 and N2 are 32 and 28, respectively. 1. Mathematical method (1) Assignment of unknown variables x = mass fraction of O2 , y = mass fraction of N2 (2) Set up equations satisfying the given conditions x + y =1, (x/32)/(x/32 + y/28) = 0.21 (3) solve the equations: x = 0.23, y = 1-x = 0.77 2. Chemical Engineering Methods Using a Basis of Calculation (1) Set up a proper basis of analysis Basis: 100 kg mol Air (2) Analyze question and given conditions in a table. component kg mol mw kg mass fraction -----------------------------------------------------O2 21 32 672 672/2900 N2 79 28 2228 2228/2900 -----------------------------------------------------Total 100 2900 (3) Answer the question from the table. mass fraction of O2 = 672/2900 = 0.23 mass fraction of N2 = 2228/2900 = 0.77 1