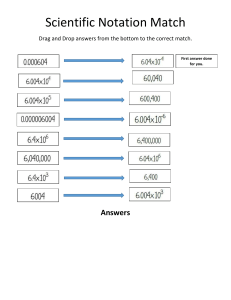
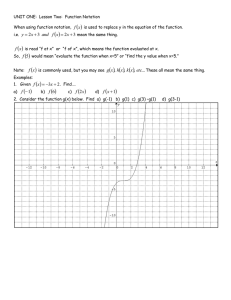
AS Chemistry 14/03/2024 Electronic Configuration The Aufbau Principle and Hunds Rule (‘bus rule’) will be tested in principal as explained below. Electrons are filled up using the: 1s 2s etc notation and/or electron in a box notation N.B: arrows represent the filling order for EACH subshell (Aufbau Principle) Complete the following table using the 1s2s notation: Atom/ion No. of electrons FULL electronic configuration Oxygen Chlorine Magnesium ion Oxide Ion Nitride Ion Neon Carbon Sodium Hydrogen Ion Some of Atoms and Ions have the SAME electronic configurations. SUGGEST a term used to explain this? …………………………………………………………………………………………………………………. 3rd Principal Quantum Number i.e 3rd Shell Electronic configuration of the elements that fill electrons in the 3rd shell, THREE principles must be considered. 1) The Energy levels for the 4s and 3d subshells (when using the 1s 2s notation) 2) When writing the electronic configuration for IONS - AWLAYS remove the 4s electrons FIRST (applies to the first row of the transition elements) 3) ‘Stable’ configurations when looking at Electron (when using the electron in the box notation – ONLY exceptions tested in the exam are for Cr & Cu ATOMS, this is not in accordance with the Aufbau principle) Now complete the FULL electronic configurations of the following, using the 1s 2s notation. DO NOT DO Chromium and Copper Atom/ion Scandium Bromine Copper Copper ion Bromide ion Iron Iron (II) Iron (III) Chromium Chromium (III) No. of electrons FULL electronic configuration