Introductory Physics Lab Manual: Mechanics, Oscillations, Thermo
advertisement

Contents
Introduction: How to Succeed in Physics Lab
Tutorial 1: Errors and Significant Figures
Tutorial 2: Making a Good Graph by Hand
page
3
5
9
Laboratory Exercises
Mechanics
1
2
3
4
5
6
7
Density; Significant Figures
Position and Velocity
Acceleration
Vectors
Tension Force and Acceleration
Conservation of Energy and measuring g
Collisions and Momentum
Oscillations
8
Part I: Simple Harmonic Motion
9
10
Part II: The Simple Pendulum
The Sonometer
Properties of Sound Waves
Thermodynamics
11
12
13
The Mechanical Equivalent of Heat
Boyle's Law
Part I: Heat of Fusion of Ice
Part II: The Specific Heat of a Metal
1
This collection of Laboratory Exercises is the introductory physics laboratory manual used
by Hunter College. The original exercises were developed by the Physics Faculty over thirty
years ago. A number of revisions have since been made. In particular, major revisions led
by Professors Robert A. Marino in 1994 and by Mark Hillery and Y.C. Chen in 2002,
introduced several new exercises involving modern electronic and optical equipment and
computerized data acquisition systems. We are indebted to the faculty and students who
participated in the creation and revision of the manual over the years.
Physics Faculty
Hunter College
June,2002
© 1998, 1999, 2002 by
Department of Physics, Hunter College of the City University of New York
All rights reserved.
2
INTRODUCTION
How to Succeed in Physics Lab
Read the lab manual before coming to class to become familiar with the experiment.
Lecture and Lab are NOT in perfect synch, so you may have to give the textbook a look
also.
You should take responsibility to learn safe operating procedures from the lab instructor.
The lab manual is also occasionally a good source of safety tips. With electrical circuits, no
power is to be supplied unless OK'd by instructor or lab tech. Report any accidents
immediately!
You will work with a lab partner to take data, but you are individually responsible for your
own data. All subsequent calculations, graphs, etc. are also your own individual
responsibility.
Original data MUST be in ink. If you change your mind, cross out with a single stroke, and
enter new datum nearby.
Do not leave the lab room without obtaining the instructor's signature on your original data
sheet. Without it, your lab report will not be accepted. No exceptions.
The lab has been designed to be a "low pressure" experience. We hope it is an enjoyable
one as you take the time to become familiar with new equipment and experiences. Still,
you should aim to complete all data-taking, all necessary calculations, reach all conclusions,
and at least sketch all graphs before you leave. It's well known (to those who know it well)
that once you walk out that door, all work on lab reports will take longer. Besides, most of
the grade for the course will come from the lecture part, so spend your time accordingly.
Before taking good data, run through the experiment once or twice to see how it goes. It is
often good technique to sketch data as you go along, whenever appropriate.
The Report:
Your Lab Report should be self-contained: It should still make sense to you when you pass
it on to your grandchildren. It should include:
a) Front page: your original data sheet with your name, partners and date. The original data
MUST be in ink. Report not acceptable if original data is in pencil or if data sheet was not
signed by your instructor. (So... don't leave lab room without it.)
b) Additional pages with data and calculations in neat tabular form. If the original came out
messy, you should rewrite your data before continuing with calculations.
c) Any graphs. Neatness counts! It's one of the aims of this lab to produce students that
know how to produce a decent graph.
d) Answers to any Questions
3
e) An Appendix made up of the pages from the lab manual that describes the experiment.
Including them relieves you from having to rewrite the essential points of the procedure,
description of equipment, etc.
Lab reports are due the next time the lab meets. At the beginning of the period! It is
department policy to penalize you for lateness in handing in lab reports. This is to
discourage you from working on stale data with the lab experience no longer fresh in your
mind. A schedule will be announced.
Laboratory Grade: The lab instructor will make up a grade 90% based on the average of
your lab reports, and 10% on his/her personal evaluation of your performance in the
laboratory. This grade is then reported to your lecturer for inclusion in the final course grade
(15% weight factor). The list below will give you an idea of the criteria used by your lab
instructor in grading your lab report:
1. Quality of measurements. Logical presentation of report contents.
2. Accuracy and correctness of calculations resulting from proper use of data and
completion of calculations.
3. Orderly and logical presentation of data in tabular form, where appropriate.
4. Good-looking graphs, easiness to read, good choice of scales and labels.
5. Comparison with theory.
6. Answers to Questions; Conclusions.
7. Clarity, Neatness, Promptness.
4
TUTORIAL # 1
On Errors and Significant Figures
Errors
We could distinguish among three different kinds of "errors" in your lab measurements:
1. Mistakes or blunders. We all make these. But with any kind of luck, and some care, we
catch them and then repeat the measurement so that these errors can be corrected.
2. Systematic Errors. These are due either to a faulty instrument (a meter stick that shrank
or a clock that runs fast) or by an observer with a consistent bias in reading an instrument.
These errors affect all of the measurements in the same way, i.e. they make them all too
big or too small. For example, if our clock runs fast, all of our measurements of time
intervals will be too long.
3. Random Errors. Small accidental errors present in every measurement we make at the
limit of the instrument's precision. These errors will sometimes make a measurement too
big and sometimes too small. To deal with these we repeat a measurement several times
and take the average. The average gives the best estimate of the quantity we are trying to
measure, and the spread of the values about the average gives us an estimate of the
uncertainty.
After blunders are eliminated, the precision of a measurement can be improved by reducing
random errors (by statistical means or by substituting a more precise instrument, i.e., one
that yields more significant figures for the same measurement.) Accuracy can be increased
by reducing any systematic errors as well as by increasing the precision.
Significant Figures, Error, Fractional Error
No measurement of a physical quantity can ever be made with infinite accuracy. As an
honest experimentalist, you should relay to the reader just how good you think your
measurement is. One simple way to relay this information is by the number of significant
figures you quote. For example, 3.4 cm says one thing, 3.40 cm tells a different story. The
last digit you write down can be your best estimate made between the markings of a scale,
but it still represents a willfully reported number, it still is a significant figure.
The placement of the decimal point does not change the number of significant figures. For
example, 20.8 grams and 0.00208 grams each have three significant figures; each is
assumed to be uncertain by at least ±1 in the last figure, i.e., ±1 part in 208.
Normally, figuring out how many significant figures are in a stated number gives no
problems, except when zeros are involved. For example, is it obvious how many significant
figures are expressed in 5500 feet, 250 years, or $1,300,000? A good way to tell the reader
which is, in fact, the last significant figure is by using scientific notation. For example, 5.50
x 103 feet, 2.5 x 102 years, and 1.300 Megabucks, telegraph that the number of digits in
which any confidence can be placed was three, two, and four, respectively.
5
Another way of representing the error is to explicitly indicate it. Suppose that we measure
a length, which we find to be 1.520 m, and we believe that this measurement is accurate
to within a centimeter. We could then write the result of our measurement as 1.520±0.005
m, which means that our result is between 1.515 m and 1.525 m. In general, if we are
measuring a quantity x, we can write the result of a measurement of x as xbest±Δx, where
xbest is our best estimate of the quantity being measured, and Δx, which is a positive
number, is the value of the uncertainty in our measurement. We can also use what is
called the fractional error. This is just Δx /| xbest |. To get the percent error, we just multiply
the fractional error by 100. In our example, we have that xbest =1.52 m, Δx =0.005 m, the
fractional error is 0.0032 and the percent error is 0.32. Note that the fractional error and
the percent error have no units.
Finding the uncertainty
We have been discussing representing the result of a measurement as xbest±Δx, but given
a set of readings from an instrument, how do we find xbest and Δx? We find xbest by taking
the average of the readings, so if we have made three measurements of a length, and have
found the results 1.26 m, 1.28 m and 1.25 m, we have that xbest = (1.26 m + 1.28 m +1.25
m )/3 = 1.26 m. Finding Δx is a bit trickier. First, it cannot be any smaller than the precision
of our measuring instrument. If we are using a meter stick to measure a length, and the
smallest units marked on the meter stick are millimeters, then the uncertainty in our
measurements will be approximately one millimeter (it can actually be a bit less than this,
because we can estimate the length to a fraction of a millimeter – this would give us an
uncertainty of about 0.3 mm). However, it might be bigger due to random errors. In order
to do a detailed analysis of random errors, it is necessary to know a lot more about
probability theory and statistics than you do now, so we will just give you the answer.
Suppose you have made N measurements and the results are x1, x2, …xN. You first
calculate the average value of the measurements, xbest, and then you use it to find what is
called the standard error, s, which is given by s = [(x1 - xbest)2+(x2 - xbest)2+…(xN xbest)2]1/2/[N(N-1)]1/2. The uncertainty, Δx, will just be the sum of the uncertainty due to the
measuring instrument and the standard error, s.
Computations using raw data
How do you combine your carefully gathered data with other numbers in an expression?
With a little common sense, and a hand calculator, you can verify that the following rules
should be followed:
Addition and Subtraction:
Suppose we want to add two measured lengths, 0.36±0.01 m and 0.48±0.02 m. Why would
we want to do this? Each length might be the length of a block, and then the sum would
correspond to the length of the object we get when we put the two blocks together. What
can we say about the length of the combined blocks? Our best estimate of their combined
length is just 0.36 m + 0.48 m = 0.84 m, but we have to worry about the uncertainties too.
The longest the combined blocks can be is (0.36 m + 0.01 m)+(0.48 m + 0.02 m) = 0. 87 m,
and the shortest they can be is (0.36 m - 0.01 m)+(0.48 m - 0.02 m) = 0. 81 m. We can
then write the combined length as 0.84 ± 0.03 m.
6
This gives us the following rule. Suppose that q=x+y, and that x and y are measured
quantities, which we represent as xbest±Δx and ybest±Δy. Then qbest= xbest + ybest and
Δq=Δx+Δy. The same rule holds for subtraction, if q = x-y, where x and y are measured
quantities, then qbest= xbest - ybest and Δq=Δx+Δy.
One conclusion that can be drawn from this is that when adding numbers with different
numbers of significant figures, the number with the least number of significant figures
determines the number of significant figures in the sum. For example, 3.1 m +1.11 m is 4.2
m, and not 4.21 m. Similarly, 1.11 x 103 m + 3.33 x 104 m is, unfortunately, just 3.44 x 104
m.
Note: To see this you have to write it out in ordinary notation (even better: line-up one under
the other):
1,110 + 33,300 = 34,410 mathematically
but the tens position is not significant in one of the terms, so it cannot be significant in the
final sum. The answer is 34,400, or 3.44 x 104.
Multiplication and Division:
Suppose we have measure the sides of a rectangle and we want to find its area. In
particular, we found the length to be 0.240±0.002 m and the width to be 0.120±0.001 m.
Our best estimate for the area is just Abest=(0.240 m)(0.120 m) = 0.0288 m2, but what is the
uncertainty? The biggest A can be is (0.242 m)(0.121 m)=.0293 m2, and the least it can be
is (0.238 m)(0.119 m) =0.0283 m2, so ΔA=0.005 m2. Can we use this example to come up
with a rule? Let us look at the fractional errors. The fractional error for the length is (0.002
m)/(0.240 m)=0.0083, the fractional error for the width is (0.001 m)/(0.120 m)=0.0083, and
the fractional error for the area is (0.005 m)/(0.0288 m)=0.172. Notice that the fractional
error for the area is approximately the sum of the fractional errors of the length and the
width, (0.0083+0.0083=0.0166). This is, in fact, a good general rule, when multiplying the
results of two measurements, the fractional error for the product is approximately the sum
of the fractional errors of the factors.
Let us see why this is true. Suppose that q=xy, where x and y are measured quantities, so
x= xbest±x and y=ybest±y. We can write q=qbest±q, where qbest=xbestybest. This means that
qbest±q=(xbest±x)(ybest±y)≈ xbest ybest± (xbesty+ybestx), where we have assumed that is
ΔxΔy small compared to everything else. If we now divide both sides by |qbest| and then
subtract qbest/|qbest | from both sides, we get that (q/|qbest |) =(x/|xbest |)+(y/|ybest |). The
same rule works if q=x/y, but we won’t show the derivation explicitly. Summarizing, we have
that the fractional error of a product of two measured quantities is just the sum of the
fractional errors of the factors, and the fractional error of a quotient of two measured
quantities is just the sum of the fractional error of the numerator and the fractional error of
the denominator.
What does this mean in terms of significant figures? Roughly there are as many significant
figures in your final answer (product or quotient) as there were in the least precise value
you used. For example,
7
3.481 x 1.75 gets reported as 6.09, not 6.092.
Of course, you should only round off the final answer. If a number is used again in another
computation, you should not round it off in between, or you may make a small but significant
error.
8
TUTORIAL # 2
Making a Good Graph by Hand
Start thinking about a nice title. e.g., " The Square of the Period (T 2) of a Simple Pendulum
vs. its Length (L)" [ A shorter title would have been even better]
Keep your axes straight: If you need to plot "A vs B", or "A as a function of B", then A is on
the vertical axis and B is on the horizontal axis.
vertical axis = y-axis = the "ordinate"
horizontal axis = x-axis = the "abscissa"
The crucial part is choosing the range and scale for each axis. Two examples:
a) 0 to 5 sec; 5 graph boxes =1 sec.
b) -300 to +200 degrees; 2 boxes =100 degrees
The range must be:
The scale should be:
just large enough to accommodate all your data, yet allow a
readable scale.
spread out enough so your data take up most of the graph area,
and labeled so plotting (and reading) is easy.
Label the x and y axis with the appropriate magnitudes. These should be round numbers
which cover the entire range of values that you will be plotting. A common mistake is to
label too many boxes. If you are trying to show that one quantity is proportional to another,
or if you are not told otherwise, zero is part of the range and must be located at the Origin.
The numbers should be evenly spaced with the same number of boxes between the same
increase in numbers including the space from zero to the first non-zero number. Choose
an appropriate number of boxes between numbers. It is better to have 5 boxes between
numbers than 4 since it is easier to interpolate in the first case than in the second. (Similarly
10 is better than 8, and 2 is better than 3.)
Plot the results of your measurements on this graph. Where appropriate, you should include
error bars to indicate the uncertainty in your measurements. These error bars are not of
arbitrary size but should be of the size of your uncertainty on the scale dictated by the
numbers on the axis of your graph.
When you draw the line that best fits your data, the line should be a smooth one that need
not go through any points. In general, there should be as many points on one side of the
line as on the other. If you have done your work properly, the line should pass inside of the
error bars for each point. (If it does not, that may be an indication that there is something
wrong with the point in question. Perhaps you miss-recorded a measurement, or your
estimate of the error was too small, or there was something wrong with the apparatus, or
with the technique you applied, etc.) If your graph shows that one quantity is proportional
9
to another, it should be a straight line that starts at the origin and passes through the plotted
data with as many points on one side as the other.
If you are asked to find the slope of the line, choose two points on the line which are as far
apart as possible. This will minimize the error that is introduced in reading the value of those
points. The slope is the difference between the vertical values of those points divided by
the difference in the horizontal values of those points.
A common mistake is to measure the slope of the segment connecting two actual data
points: this does not yield the slope of the straight line you fitted to your data!
Note: Normally. the slope of your graphs has its own units. e.g., The slope of graph of
velocity vs. time has units of (m/s)/(s) = m/s2.
Here is a graph so messy, you can surely do better with a little practice:
10
Remember that:
Scientific experiments are done by trial and errors. Please be patient and resilient.
Please BE RESPECTFUL to the instructors, staff and your peers.
Please TAKE YOUR TIME to get familiar with all parts of the equipment.
Please be CAREFUL and GENTLE when handling the equipment.
Please DO NOT OVER TIGHTEN the screws.
Please RETURN the equipment to its original place after use.
Please TURN OFF the computers and the power supply of the equipment.
Please MAKE SURE your lab bench is CLEAN and TIDY before you leave (Each lab room is
equipped with paper tower and garbage can).
11
LABORATORY EXERCISE #1
Density; Significant Figures
Objectives
To become familiar with instruments to measure length (meter stick, vernier caliper,
micrometer) and to appreciate the difference between accuracy and precision of
experimental measurements. Incidentally, to learn the concept of mass density, and
practice proper use of significant figures.
Equipment and Supplies
Metal samples, electronic balance, meter sticks (long and short), vernier caliper, vernier
model, micrometer calipers.
Discussion
The mass density of a body measures the amount of mass per unit volume of that body.
Definition:
Density of a body = (body's mass)/(body's volume) or D = M / V
The density of a substance does not depend on the shape or the particular amount of that
substance. For example, the density of a small gold ring and the density of a large gold
brick should be the same number, i. e., the density of gold.
As part of your density calculations you will need to compute the volume of a regular solid
from its linear dimensions. The quality of your volume measurements will thus depend on
how precisely you can measure lengths. You will use three progressively more precise
instruments: a wooden "meter stick", vernier calipers and a micrometer. Your instructor will
demonstrate how to use each one. In each case, you should read the instrument to the
smallest division plus one more digit by estimation. So, a meter stick with millimeter
markings can be used to estimate a length to the nearest tenth of a millimeter, e.g., 12.4
mm, or 1.24 cm. This estimate by "eye" is often only good to ±2 or 3, as you can verify by
repeating the measurement or asking your partner to do the estimating. A vernier is an
invention that removes the uncertainty in reading to the nearest tenth between adjacent
markings, thus increasing the precision of the final measurement. Your instructor will
demonstrate how this is done with the vernier model, prominently displayed in the front of
the laboratory room.
On Errors and Uncertainty in a measurement:
When you work out a math computation, the numbers are usually considered exact, e.g.,
1.1 x 1.2 x 1.3 = 1.716. But when a number represents a physical measurement, it is never
exact because of the limitations of the instrument used, or the way it was employed, etc. It
is essential, therefore, that each experimental result be presented in a way that indicates
its reliability. A very simple way to do this is by the use of significant figures. (See Tutorial
#1, on Significant Figures)
12
As an example, consider how different the following three cases are, even though they refer
to exactly the same steel block:
a) 1.1 cm x 1.2 cm x 1.3 cm = 1.7 cm3
b) 1.13 cm x 1.20 cm x 1.29 cm = 1.75 cm3
c) 1.127 cm x 1.195 cm x 1.293 cm = 1.741 cm3
What is different about these three reports, is the precision with which the data was taken.
(By the way, all three workers used significant figures correctly.)
Now, how accurate are the results? This concept reports on how close the reported answer
is to the "accepted answer". What determines accuracy? Examples are the calibration of
the measuring instruments or systematic errors on the part of whoever is taking the data.
The following somewhat oversimplified table may be useful in thinking about these
concepts:
Problem
Remedy
_______________________________________________________________________
Mistakes and blunders
Repeat measurements several times to check on yourself
Systematic errors
Random errors
Use calibrated instruments, use them properly
Treat data statistically and report on the average
magnitude of errors
Procedure
A. Estimating the number of kilograms of air contained in this room when it is empty.
Estimate the volume of the room by the following procedure. First measure the height,
width and length ignoring protruding structural columns. Use the long meter sticks, and
make full use of the geometric tiles on the floor (they are very nearly 12" x 12"). The
resulting volume yields the mass of the air when multiplied by the density, 1.29 kg/m3. Your
answer here should reflect the number of significant figures of your raw data.
B. Finding the density of a metal block. First weigh the block carefully with the balance
provided. Then, measure the dimensions of the block with three different instruments:
B1. Use of meter stick. Lay block on meter stick, measure the position of each edge,
estimating to the nearest 0.1 mm. Take several readings on different parts of the specimen.
B2. Use of vernier calipers. Examine the calipers. What is the distance corresponding to
the smallest markings on this instrument? How many significant figures can be obtained
when measuring a length between 1 and 10 cm? Is the "zero" calibrated correctly, or do
you need to correct for a misalignment? Take several readings on different parts of the
specimen, estimating to 0.01 mm.
13
B3. Use of micrometer calipers. Examine this fine instrument, noting the proper use of the
slip clutch to prevent forcing the gears. What is the distance between smallest markings on
the shaft? Open the jaws to the 1-mm mark. Rotate the micrometer thimble through two
revolutions: record the reading. Rotate by one revolution. Record the reading again.
Careful consideration of your data should lead you to understand how the micrometer can
be read correctly to 0.001 mm. When you are satisfied that you can use and read this
instrument, obtain several readings of each of the three dimensions of the metal block. Note
any misalignment of the "zero" and correct your data accordingly.
C. Repeat procedure B for a cylindrical metal block, time permitting.
Calculations and Conclusions
A. Complete and sign the statement in the Data Sheet.
B. For each of the three methods in part B, compute the density of the metal block to the
appropriate number of significant figures.
1. Are the three results consistent with one another? What criteria did you use to answer
the question?
2. For your best value of the density, compute the percent deviation from the accepted value
(obtained from your instructor).
Note on the "propagation of errors":
The equation for the volume of a rectangular solid is V l w h Applying our rule
for products, that means that the fractional error of the volume is just the sum of the
fractional errors of the for the length, width and height. You can use this to find the
uncertainty, ΔV, in the volume itself.
V
(l ) 2 (w) 2 (h) 2
2
V
l2
w2
h
*Just
ONE significant figure is appropriate, here.
14
LABORATORY EXERCISE #1
Data Sheet: Density; Significant Figures
Name:______________________________
Date:_________
Partners:_________________
Instructor's signature:____________________
A.
I have measured the volume of the air in this laboratory room and hereby report
that using an assumed density of ____________ kg/m3, I find that the room
contained _________ kg of air. {How close you come to the "true" answer is how
accurate your measurement was.}
I certify that, should another careful
experimentalist attempt to make the same measurement, she (he) will come within *
± ________%, approximately, of my value. {Too high a number here, will show you
to be a sloppy experimentalist, unable to squeeze the best from the tools available.
Too low a number will expose you as a fraud, claiming a precision unwarranted by
your instruments and methods of using them.}
________________________
(signed)
B. For each of the three methods in part B, compute the density of the metal block to the
appropriate number of significant figures. Present data, calculations and answers below.
Use additional sheets if necessary.
15
B1. Are the three results consistent with one another? What criteria did you use to answer
the question?
B2. For your best value of the density, compute the percent deviation from the accepted
value (obtained from your instructor).
16
Post-Lab Test: Density; Significant Figures
Name:__________________________
Complete the homework problems on this sheet and include with your Lab report.
Problem 1.
A block of wood has dimensions 10.20 cm by 10.15 cm by 2.45 cm. What is the volume of
this block in units of cubic centimeters? (By all means, use your calculator.)
a) 253.6485
b) 253.65
c) 253.7
d) 254
Answer:__________________
Now, if the mass of the block is 175.2 g, find the density.
Density = _________________ g/cc
Assume that the accepted value is 0.665 g/cc. Determine the percent deviation of your
result from the expected value. { cm3 = cc}
Percent Deviation :____________________%
Note: Percent Deviation is the difference between your value and the accepted value times
100 then divided by the accepted value.
In this course we shall report percent deviations to at most two significant figures.
Problem 2.
A student measures the mass of 49.4 cc of water and finds the mass to be 50.2 g. Assume
that the accepted value is 1.00 g/cc. Compute the density of the water and the percent
deviation.
Computed Density = ___________________
Percent Deviation = ______________%
Remember to include the units with the density.
Note: If you did these problems correctly, your value of density should have
agreed with the expected value to within four percent, in Problem 1. In Problem 2,
on the other
17
Laboratory Exercise #2
Position and Velocity
With an Introduction to Graphical Analysis Software
Objectives: To understand the relation between position and velocity, and to understand
how to represent each of them graphically; To familiarize with the tools used in mechanics
experiments.
Equipment and supplies: Sensor cart (green), track with end stop, computer, Graphical
Analysis software.
Computer and software
In this semester we will do a series of experiments to explore kinematics, dynamics,
momentum, and energy using one or two sensor carts on a track. The data of motion will
be collected by wireless sensors and analyzed by a computer. We will first learn how to
connect the sensors to your computer.
A. How to connect the cart sensors to the computer.
1. Place the green sensor cart on the track. The arrow should points to the end stop.
Disconnect the charging cable from the cart if it has not yet been disconnected.
2. Open Graphical Analysis program.
When the window opens up,
a. Click “Sensor Data Collection”.
b. Select the sensor number that matches the number on your green cart. Then click
“Done”. Two graphs, the Position graph and Velocity Graph, will appear on the
screen.
(If you do not see the correct sensor number, press the switch on the side of the
cart to turn it on.)
c. We will need only one plot only. To do this, click “View Options” at the upper right
corner. Choose “One Graph”.
d. Set duration of data collection
Click “Mode” on the lower left corner to open the Time Based window. Under “End
Collection”, type in 15 seconds. Then click “Done”. This will set the total time for
data collection to 15 seconds.
e. Zeroing the position axis
Move the cart to the starting point at the opposite end of the track from the end
stop. Click “Position” on the right lower corner and click zero. This will make
current position on the track to be position zero.
B. Plotting Position-Time Graph with hands.
1. Starting at the far end of the track from the end stop use your hand to move the cart
slowly and steadily toward the end stop. Practice this motion and try to complete the
motion through the track in approximately 15 seconds. Sketch the position-time graph
(1) for this motion in the report sheet and label the axes with appropriate scales and
units.
18
2.
3.
4.
Repeat, but this time move a little faster to complete this motion in 5 seconds. Sketch
graph (2).
Make a position-time graph by moving the cart from the end stop toward the other end
slowly and steadily and complete the travel in 15 seconds. Sketch graph (3).
Repeat Step 3, but this time complete the motion in 5 seconds. Sketch the graph (4)
in the report sheet.
Note: When you sketch these graphs, it is not crucial to include all the fine details.
However, be sure to label and number the axes as they appear on the screen, and
provide a title (e.g. Position vs. Time.).
5.
6.
Sketch your prediction of the position-time graph on graph (5) produced by the
following: Starting from the left end of the track, move slowly and steadily to the end
stop for 5 seconds, stop for 5 seconds, then move more quickly and steadily toward
the left for 3 seconds.
Now test your prediction with the data collected by the computer. Click “COLLECT”
and carry out the motion sequence in Step 5. You may repeat this procedure. When
you are satisfied with the outcome, take a screenshot with your mobile phone.
(Does your result match your prediction in Step 5? If not, make the necessary adjustments
to your prediction and/or your motion until the graphs agree).
7.
Now let us see if we can do the reverse, given the following graph we want to move to
match it.
Move the cart in such a way to make your data look as much like the above plot as
possible. Take a screenshot for the best graph you can produce.
C Velocity-Time Graphs
We will examine how velocity varies with time for different types of motion. We can do this
by generating velocity-time graphs.
19
We will repeat Steps in B 1 - 4, but this time make corresponding velocity-time graphs (a)
to (d) in the report sheets.
D Position and Velocity Graphs
We now want to study the relation between position and velocity. We will measure the
position and velocity of a moving cart simultaneously. This will give us better data.
1. Open two graphs
Click “View Option” on the upper right corner. Select “Two Graphs”.
You should see two sets of plots on the screen, a position-time graph and a velocitytime graph.
2. Practice the following moves: Place the cart at the opposite end of the track from the
end stop. Gently push the cart to set the cart n motion and stop the cart before it hits
the end stop. Avoid using too much force which can cause damages to the cart.
Exert appropriate force to let the cart travel the entire length of the track in about 3
seconds.
3. When you are comfortable with the execution, click “COLLECT” and push the cart and
observe the distance and velocity data being plotted on the screen.
4. Click the magnifying glass on the lower left corner of each plot to enable auto-scaling to
make the curves filling the graph. Take a screenshot.
5. Reading data from the graphs
a. The readings of position and velocity at a given time can be read by moving the
cursor along the time axis.
b. Click “Graphic Options” at the lower left corner of the Position-Time graph. Enable
“Tangent”. The graph will display the reading of the slope of the curve. Record the
positions and slopes at five different points along the time axis.
c. To find the average value of velocity between time t1 and t2, highlight the
segment of data between t1 and t2, click “Graphic Options” and select “View
Statistics”. The mean value of velocity will be displayed in a pop-up
window. Compare to the average value of the slope in the position-time
graph in mean value of velocity.
6. Comparing manual and automated measurements
Next we compare the velocity on the graph with the velocity obtained the old fashion
way using a ruler and a stopwatch. We will first determine the velocity by measuring
the time it takes for the cart to travel through a distance.
a. Turn on the stopwatch function in your mobile phone. Set the cart in motion from
the opposite end of the track toward the end stop and measure the time duration
for the cart to travel between the two red marks on the track. Practice this
operation several times until you feel comfortable with your execution. When you
are ready, zero the initial position, set the cart in motion and measure the time of
travel using your stopwatch, while your partner simultaneously clicks “COLLECT”
to start data collection. Record the stopwatch reading t and the positions x1 and
∆𝑥
x2 of the red marks. Calculate the average velocity ( 𝑣̅ = ∆𝑡 ) between the two red
marks.
20
b. Next find the average velocity from the graph. This can be done by recording,
from the position graph, time t1 and t2 when the cart passes the red marks on the
track and find the average velocity during this period from the Velocity graph using
View Statistics Function. Compare the velocity measured manually in (a) with the
average velocity reading from the graph. Present a screenshot of the graph using
a mobile phone.
E. Verifying the equation 𝒙𝟐 = 𝒙𝟏 + 𝒗(𝒕𝟐 − 𝒕𝟏 )
7. Suppose we want to find position information from a velocity graph. If an object is
moving with a constant velocity, then the distance it travels between two times, t1 and
t2, is just the velocity measured at t1 multiplied by (t2-t1). We can see if our graphs
confirm this.
Set the cart in motion and start collecting data. Zoom in all graphs. Take screenshot.
Choose two times in the region where the velocity is nearly constant, which we shall call
t1 and t2, and find the positions corresponding to them using the position-time graph. Let
us call these positions x1 and x2. Read the velocity at t1. Calculate x2.
Compare the calculated x2 with the measured value.
8. Repeat Step 7 for 2 more pairs of t1 and t2.
At the end of this experiment, re-connect the charging cable to the
cart and place the cart on the track.
21
Laboratory Exercise #2
Position and Velocity
Date ______________
NAME:______________________________Partners:________________________
Position-Time Graphs
Position (m )
(2) Faster and steady motion to the end stop
Position (m )
(1) Slow and steady motion to the end stop
Time (s)
Time (s)
Position (m )
Position (m )
(3) Slow and steady moving away from end stop (4) Faster and steady moving away from end stop
Time (s)
Time (s)
22
POSITION. ( m)
(5) Prediction
TIME ( s )
(6) Screenshot of position- time graph which corresponds to the prediction in (5).
(7) Paste the screenshot that reproduces the Position-Time graph shown in
Section 7.
23
Velocity-Time Graphs
(b) Faster and steady moving toward end stop
Velocity (m/s)
Velocity (m/s)
(a) Slow and steady moving toward end stop
Time (s)
Time (s)
Velocity (m/s)
Velocity (m/s)
(c) Slow and steady moving away from end stop (d) Faster and steady moving away from end stop
Time (s)
Time (s)
24
D Position and Velocity Graphs
Provide a screenshot for position and velocity graphs obtained in D.
Data from the position-time graph:
Record five points (with units) from the position-time graph, calculate the average
value.
Position, x
Time, t
Velocity
x
(slope)
t
Average velocity =
Comparing the average velocity with the average velocity reading from the View
Statistics function.
25
D6
Compare manual and automated measurements
Positions of red marks
X1=___________________. X2=__________________________
Stopwatch reading: ____________________________________
Average Velocity=_____________________________________
Provide a screenshot of the position- and velocity-graphs.
Deduce the average velocity from the graph and compare with the average velocity
obtained manually.
26
̅ (𝒕𝟐 − 𝒕𝟏 )
Verifying the equation 𝒙𝟐 = 𝒙𝟏 + 𝒗
Paste the screenshot to be used for the analysis.
Use the position, x1, corresponding velocity, v, at a given time, t1, to predict the
position x2 at time t2 using the equation 𝒙𝟐 = 𝒙𝟏 + 𝒗(𝒕𝟐 − 𝒕𝟏 ) . Find the x2 values
for three different t2.
Compare the predicted x2 with the readings from the position-time graph. If the
predicted values do not match the experimental values, explain why.
27
LABORATORY EXERCISE #3
Acceleration
Objectives:
To study the relation between position, velocity, and acceleration when the acceleration is
constant.
Equipment:
Sensor cart, track with end stop, 10-g, 20-g cylindrical masses, pulley, strings.
Introduction:
Velocity tells us how position changes with time, and acceleration tells us how velocity
changes with time. If an object, like a cart, has a constant acceleration, then we can find
what the acceleration is by calculating Δ𝑣/Δ𝑡, where the velocity of the object changes by
an amount 𝛥v in a time interval 𝛥t. The relation between acceleration and position is
more complicated. In particular, the position of an object with constant acceleration
depends not only on the acceleration, a, but on the initial velocity, v0 and initial position, x0,
1
of the object as well. The exact relation is 𝑥(𝑡) = 𝑎𝑡 2 + 𝑣0𝑡 + 𝑥0 .
2
A. Velocity and Acceleration
Our first task is to understand the relation between velocity and acceleration. We will
again use the cart on a track, but this time there will be weight attached to the cart to
make it accelerate. First we will look at the velocity and acceleration of the cart when it is
speeding up, and then we will look at them when the cart is slowing down.
1. Set up the cart on the track with the pulley attached to the end of the track. The 20-gram
weight is then attached to the cart through a string which is laid over the pulley.
2. Open Graphical Analysis software. Connect the sensor to the computer. You should
see two graphs on the screen. Change the vertical labels to velocity-time graph,
acceleration-time graph.
3. What you are going to do is to move the cart to the far end of the track with the weight
lifted to the height of the pulley, zero the position, click Collect and release the cart. The
position sensor will collect data while the cart is moving. Before you do this, sketch in
graphs (1) and (2) a picture of what you think the velocity-time graph and acceleration-time
graphs will look like (hint: the weight gives the cart a constant acceleration). Now do it and
see how the actual graph and your prediction compare. When you do the experiment, hold
on to the cart until you are ready to take the data, and then release it (do not give it a push).
Catch the cart before it hits the pulley.
4. Read off four pairs of points from the velocity-time graph. Calculate
(Δ𝑣/Δ𝑡) = (𝑣2 − 𝑣2 )/(𝑡2 − 𝑡2 ) for each pair, and find their average value. Now use the
28
View Statistics feature to read average acceleration and compare to the computed average
values in the corresponding time range.
5 Deceleration (negative acceleration)
a. Now we want to try something a bit different. You are now going to start the cart with
the 10g weight laying over the pulley and hanging in the air, give the cart a gentle push
away from the pulley. (Pushing too hard can result in the weight hitting the pulley and make
the analyze difficult.) The cart will move away from the pulley, decelerate, turn around and
come back. Before you do this, sketch a prediction for the velocity-time and accelerationtime plots in graphs (3) and (4). Pay particular attention to what you think is going to happen
to the velocity and the acceleration at the turning point.
b. Before taking the data, we need to re-define the positive direction because the initial
velocity of the cart is in the opposite direction of the arrow. Click Position on the lower right
corner of screen, enable Reverse.
Then Click Collect. Repeat this procedure, if necessary, until the velocity-time graph has
at least two seconds of straight line. Click the magnifier to zoom in. Take a screenshot.
B. Position, Velocity and Acceleration
We now want to understand how position is related to acceleration and velocity when there
is a constant acceleration.
1. Click View Options to choose 3 Graphs. Change the vertical labels to display Position-,
Velocity, and Acceleration graphs. From class we know that the velocity will be a linear
function of time, (𝑡) = 𝐴𝑡 + 𝐵 , and the position should be a quadratic function of time,
𝑥(𝑡) = 𝐴𝑡 2 + 𝐵𝑡 + 𝐶. We want to figure out what those functions are for the data in our
last run. The coefficients that appear in those functions are related to the acceleration,
initial velocity ( at t=0) and the initial position (at t=0).
2. Choose a segment in the velocity-time graph, which should look something like a straight
line, Apply Curve Fit (under Graph Options). What physical quantities do A and B
represent? Is either one related to your acceleration graph? If so, compare the value you
found for A or B to the relevant value on the acceleration graph.
3. Now go to the position-time graph. We want to find an equation for this curve, which
should be something like a parabola. This means we should have, 𝑥(𝑡) = 𝐴𝑡 2 + 𝐵𝑡 + 𝐶.
We want to find the coefficients A, B, and C. We can do this by using the Quadratic Fit
under View Statistics Leave the fitting curves on the screen and take a screenshot.
Read off the values of A, B, and C. What physical quantities do A, B, and C represent?
Are any of them related to either your acceleration graph or to your velocity-time graph? If
so, compare the values you found here to the values you found from your acceleration and
velocity-time graphs.
29
LABORATORY EXERCISE #3
Acceleration
Date ______________
NAME: _____________________________Partners: ________________________
Instructor's Signature: ___________________
Velocity and acceleration
Velocity (m/s)
Acceleration (m/s/s)
Predict the velocity-time and acceleration-time relations when the cart is pulled by
the weight.
(1)
(2)
Time (s)
Time (s)
Paste the screenshot for the velocity and acceleration graphs for empty cart.
30
Read off four pairs of points from the velocity-time graph. Calculate
for each pair,
Velocity, v
Time, t
Δ𝑣
Δ𝑡
=
Slope a
𝑣2 −𝑣1
𝑡2 −𝑡1𝑡
v
t
Average value=
Average of four acceleration values from the acceleration-time graph =
How does the values of average acceleration from the acceleration-time graph compared
to the average values of (Δ𝑣/Δ𝑡)?
31
Velocity (m/s)
Acceleration (m/s/s)
A5
Sketch predicted velocity-time and acceleration-time graphs for the procedures
outlined in step (5).
(3)
(4)
Time (s)
Time (s)
Paste the screenshot obtained in 5 b with the cart pulled by 10g over the pulley.
Compare the measured with the predicted graphs. Use your own words to explain
why the graphs make sense.
Use Apply Curve Fit over a linear segment in the velocity-time curve with the
equation v=At+B and find A and B.
What physical quantities do A and B represent? Is either one related to your
acceleration graph? If so, compare the value you found for A or B to the relevant
value on the acceleration graph. What are the physical meanings of A and B.
32
B. Paste the 3 graphs screenshot here.
Fit the position-time curve with 𝑥(𝑡) = 𝐴𝑡 2 + 𝐵𝑡 + 𝐶 and read off the values of A, B, and
C. What physical quantities do A, B, and C represent? Are any of them related to either
your acceleration graph or to your velocity-time graph? If so, compare the values you
found here to the values you found from your acceleration and velocity-time graphs.
33
LABORATORY EXERCISE #4
Vectors
Objectives:
To learn adding vectors by their components and to compare the calculation with an
experiment.
Equipment and Supplies
Force table, three low-friction pulleys, mass hangers, mass set.
Discussion
Consider a vector A that lies in a plane. It can be expressed as the sum of two components.
The components are usually chosen to be along two perpendicular directions. An example
is shown in Figure 1. The vector A can be resolved in to its x- and y-components by drawing
two lines perpendicular to the x- and y-axes. Then Ax and Ay are the x and y components
of A, respectively. From Figure 1, Ax=A cos and Ay =Asin .
y
A
Ay
X
Ax
Figure 1
Consider then the addition of two vectors A and B to give a resultant vector C, as shown in
Figure 2.
y
B
C
b
A
c
a
34
X
Figure 2
C=A+B
(1)
The x and y components of these three vectors satisfy the following relations:
Cx = Ax + Bx
(2)
Cy = Ay + By
(3)
where
Ax=A cosa
(4)
Ay =Asin a
(5)
Bx=B cosb
(6)
By =B sinb
(7)
The magnitude and angle of C can be determined by:
C=( Cx2+ Cy2)1/2
(8)
c= tan -1(Cy / Cx)
(9)
In the present experiment, the methods of vector addition is used to study the condition of
equilibrium for three forces on a force table. These forces are created by the weights
attached to the mass hangers. The diagram of the vectors involved is illustrated shown in
Figure 3.
A
C=?
B
Figure 3
When the sum of three is zero, the knot is at the origin. We have
A+B+C = 0
(10)
35
Forces A and B are created by the weights of two known masses. To determine the
unknown force C, we can rewrite Equation (10) in the following form:
C= -(A+B) =(-A) + (-B)
(11)
Then the method described above can be directly applied to determine C.
Procedure
A Set up the force table on a level plane. Attached three pulleys on the rim of the round
table.
B. Hang the following masses on two of the pulleys and clamp the pulleys at the given
angles:
Force A : 20 g at 0
Force B : 10 g at 270
Place the third pulley (Force C) at 150 with 20 g on the hanger. Add masses to this hanger
until the knot is centered at the origin. Due to a small friction in the pulley, you may help
the gently tap the strings to help initiate the motion.
To correctly calculate the forces, the mass of the hanger (5 g) must also be included in each
force. The force in Newtons equals the mass multiplied by 9.8 m/s2.
C. Place two pulleys at 0 with 200 g on the hanger and 210 with 250 g on the hanger.
By trial and error, find the angle for the third pulley and the mass, which must be suspended
from it that will balance the forces. Again, tapping the strings to help
Record the angle and mass.
Calculations and Conclusions
1. Calculate the magnitudes and the angles of the unknown vectors for the experimental
conditions of A to C.
2. Tabulate the calculated and measured results.
36
LABORATORY EXERCISE #4
Data Sheet: Vector Analysis
Date:_____________
NAME:______________________________Partners:________________________
Instructor's signature:____________________
Procedure B
Force A
A
Force B
a
B
b
Force C
Experimental
C
c
Force C
Calculated
C
c
Kg
Kg
Kg
Kg
N
N
N
N
Newton = 9.8 Kg x m/s2.
Don’t forget to include the mass of the hangers into the calculation.
Show the calculation of Force C using the space below.
37
Procedure C
Force A
A
Force B
a
B
b
Force C
Experimental
C
c
Force C
Calculated
C
c
Kg
Kg
Kg
Kg
N
N
N
N
Show the calculation of Force C using the space below.
38
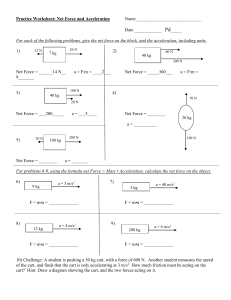
LABORATORY EXERCISE #5
Tension Force and Motion
Objectives:
To understand the relation between the total force acting on an object and its acceleration.
Equipment:
Sensor cart (green) with hook, track, end-stop, 20 g and 50 g cylindrical masses with
hooks, string, pulley wheel, and electronic balance.
Setup: Pulley at one end of track, end-stop on the other end, hook on cart.
Introduction
According to Newton’s Second Law, the acceleration of an object is proportional to the total
force acting on the it. In this experiment you will look at a situation in which the force is a
tension force. A cart on a track will have a string attached to it, and the string will go over
a pulley wheel and have a weight hanging from it. You will study the acceleration of the
cart as a function of how much mass is hanging from the string. You have to be careful,
though, because if the mass hanging from the string is m, then the tension in the string is
not mg once the cart starts to accelerate. Before you come to this lab, you should work out
the following problem:
A cart of mass M moves on a frictionless track. It is attached to a string, which goes over a
pulley wheel, and has a mass m hanging from it. At t=0 s the cart starts from rest and
accelerates. What is the tension in the string when the cart is held stationary? What is the
tension in the string when the cart is released and accelerate toward the pulley? What is
the acceleration of the cart? Express the tension and acceleration using m, M and g.
M
m
Procedure:
1. Open Graphical Analysis program. Select “Sensor Data Collection”. Select the cart
number to Connect. Under “Sensor Channels”, enable “Force” and “X-axis acceleration”
39
and disable other items. Then click “Done.
Acceleration-Time graphs.
You will see two graphs:
Force- and
3. Force sensor calibration
Place the cart on the track with the arrow pointing toward the pulley. To improve accuracy,
the force sensor needs to be calibrated.
a. Click “Force” at the lower right corner. Select “Calibration”.
b. Hold the cart and attach the string to the hook on the force probe. Run the string over
the pulley wheel and hang a 20 g mass from it. Type 0.196 N. then click “KEEP”.
type 0.49 N and click “KEEP”.
c. Hang the 50 g mass on the string and
“APPLY”. The sensor is now calibrated.
Then click
4. To verify the calibration, hold the cart and hang the 20 g mass on the string.
“Collect”. You will see the force reading on the graph to be 0.196 N.
Click
5. Hold the cart with the 20 g mass on the string, click “Collect”, and wait for three seconds
before releasing the cart. Catch the cart before it strikes the end stop. You will see the
value of the force, which is the tension force in the string, changes when the cart is released.
Use the View Statistics feature from the Graph Options at lower left of each graph to find
the average value of the tension both before and after you release the cart. In addition, use
View Statistics to find the average value of the acceleration after the cart is released. Do
this run three times and compute the average value of your results for the tension force and
the average value of your results for the acceleration.
Take a screenshot of a representative screen.
6. Repeat steps 5, but with 50 g and then with 70 g (=20 g+50 g) hanging from the string.
Find the average values of the tension force and the acceleration for each case.
7.Use the electronic balance to find the combined mass of the cart.
Plot (by hand) your force and acceleration data. On the x axis plot the acceleration and on
the y axis plot the tension force. You will have three points, one each for hanging masses
of 20 g, 50 g, and 70 g. Fit the best straight line you can to your data, and find its slope.
To what quantity should the slope correspond (assuming Newton’s Second Law is correct),
and can you compare it to any other quantity you have measured? If you can, do so. Does
your data support Newton’s Second Law?
8. If you have done the problem at the beginning of the lab, you should have a formula that
gives you the tension force in terms of the combined mass of the cart and force probe and
the hanging mass. Make a table comparing the calculated values for the tension and the
measured values.
40
LABORATORY EXERCISE #5
Tension Force and Motion
Date ______________
NAME:______________________________Partners:________________________
Instructor's Signature:____________________
Paste a screenshot of force and acceleration graphs for cart pulsed by 20 g of mass
over a pulley.
Tension before release=__________________
Tension after release=+__________________
Acceleration=__________________________
41
Record of measured tension and acceleration data of cart pulled by three different
masses over the pulley
Tension Before
Release
Tension After
Release
Acceleration
After Release
20g
Ave:
Ave:
Ave:
Ave:
Ave:
Ave:
Ave:
Ave:
Ave:
50g
70g
The mass of the cart =___________________
42
Tension Force (N)
Plot measured tension force vs acceleration. Choose suitable scales and units
and mark the axes clearly.
Acceleration(m/s/s)
Fit the best straight line you can to your data, and find its slope.
Slope=_____________________
To what quantity should the slope correspond (assuming Newton’s Second Law is correct),
and can you compare it to any other quantity you have measured? If you can, do so. Does
your data support Newton’s Second Law?
If you have done the problem at the beginning of the lab, you should have a formula that
gives you the tension force in terms of the combined mass of the cart and force probe and
the hanging mass. What is the formula? You will be asked to show and explain how you
arrived at the formula.
Compare the calculated values for the tension according the formula and the measured
values.
Calculated
Tension
20 g + mcart =
50 g + mcart =
70 g + mcart =
43
Measured
Tension
LABORATORY EXERCISE #6
Conservation of Energy
and the value of g
Objectives: To study when mechanical energy is conserved and when it is not
Equipment: Sensor cart track, friction block, electronic balance, meter stick
Figure 1 Experimental setup
Introduction
In this experiment we are going to consider two kinds of energy, kinetic energy and
gravitational potential energy, and their sum, which is the total mechanical energy. We are
going to apply these concepts to a cart moving down an inclined track. The cart will start
from rest, and then accelerate down the track. As it does, it speeds up, and its height above
the table decreases. This means that its kinetic energy increases and its potential energy
decreases. We want to see if these changes compensate each other so that their sum
stays the same. The second part of the experiment is the same as the first, except that a
friction block will be attached to the cart. We will again want to see if the mechanical energy
is constant.
From class you should remember that if an object of mass m is moving with a velocity v,
1
then its kinetic energy is given by 2 𝑚𝑣 2 . An object of mass m, which is a height h above
the origin, has a gravitational potential energy given by 𝑚𝑔ℎ, where g=9.80 m/s2. If there is
no other source of potential energy around (like a spring, for instance), then the total
mechanical energy of the mass is just the sum of the two,
1
𝐸 = 𝑚𝑣 2 + 𝑚𝑔ℎ
2
If there is no friction present, this quantity will be conserved. If there is friction present, the
mechanical energy will decrease, and we will have that
(work done by friction)=(final mechanical energy)-(initial mechanical energy)
.
44
The work done by the friction force, 𝐹𝑓 , is just – 𝐹𝑓 𝑥, where x is the distance the object
moved. Because the work done by the friction force is negative, the above equation implies
that the final mechanical energy is less than the initial mechanical energy.
Procedure
Conservation of Energy
1. Slip the spacer block(s) under the leveling feet as shown in Figure 2. (Stack multiple
blocks to form a total thickness greater than 10 cm.) Measure the distance between the
leveling feet, L, the elevation of the higher end, H, and the mass of the cart, M. Calculate
the angle 𝜃.
Place the cart on the track. The arrow should points to uphill.
Figure 2
2. Open Graphical Analysis software. Connect the sensor to the computer. You shall
see two graphs: position-time and velocity-time graphs.
Click Position at the low right corner. Choose Reverse to make downhill direction
positive.
3. You will be asked to hold the cart all the way up the ramp and then let it go. Before
doing that, sketch your predictions for the kinetic energy versus time plot, the potential
energy versus time plot, and the total mechanical energy versus time plot.
4. Now while holding the cart all the way up the ramp, click Position at the lower right
corner of the screen to Zero the position, click Collect and let the cart go. Catch the
cart before it hits the end stop.
5. Use the magnifiers to zoom in. Take a screenshot.
6. Record the position and velocity readings at five different times. Also record the
acceleration readings from the Velocity graph using Tangent under Graph Options.
45
7. Calculate the potential energy and kinetic energy and total energy and plot the energies
as a function of time. In the calculation for potential energy, g is assumed to be 9.8
m/s/s.
Compare the predicted and measured graphs and comments on any discrepancies.
Measuring g
8. So far, we assume that the value of g is the accepted value of 9.8 m/s/s. From the
vector diagram shown in Figure 3, the acceleration of cart is the component of g
along the direction of the ramp. Thus the acceleration measured in Step 6 can lead
to the value of g using the relation:
𝑎
𝑔=
𝑠𝑖𝑛𝜃
Figure 3
How does the value of g obtained this way compared to the accepted value?
Friction
9. Now attach the friction block to the cart as shown in Figure 1.
10. Lightly press the friction block to stop the cart from sliding down hill.
What is the friction when the cart is not moving?
11. You will be again asked to hold the cart all the way up the ramp and then let it go.
Sketch your predictions for the kinetic energy versus time plot, the potential energy
versus time plot, and the total mechanical energy versus time plot.
12. Now while holding the friction block, zero the position of the cart, click Collect, and let
the cart go. Use the magnifiers to zoom in. Take a screenshot.
46
Record the position and velocity readings at five different times and calculate the
potential energy and kinetic energy and total energy and plot the energies as a function
of time.
Use the fact that the change in the mechanical energy is equal to the work done by
friction to find the value of the friction force acting on the cart. This force is due to the
friction block. Use the electronic balance to find the mass of the friction block, and then
find the coefficient of dynamic friction between the friction block and the track.
47
LABORATORY EXERCISE #6
Conservation of Energy
Date ______________
NAME:______________________________Partners:________________________
Instructor's Signature:____________________
Distance between the leveling feet =_________________________
Elevation of the higher feet, H =__________________
Mass of the cart, M =___________________________
Kinetic Energy
Potential Energy
Predicted energy-time relation and explain why
Time (s)
Mechanical Energy
Time (s)
Time (s)
48
Paste the screenshot of the graphs obtained in Step 3.
Record position and velocity readings in five different times on the graphs and complete
the following table.
Table I
Time
Position
𝑥
Height
ℎ = 𝑥𝑠𝑖𝑛𝜃
Potential
Energy
=𝑚𝑔ℎ
Velocity
𝑣
Kinetic
Energy
1
= 𝑚𝑣 2
2
Total
Energy
= 𝑃𝐸 + 𝐾𝐸
1
2
3
4
5
Record the acceleration of the cart :________________________________________
49
Potential Energy (Unit:
Kinetic Energy (Unit:
)
)
Plot kinetic energy, potential energy and total energy in Table I as a function of time and
compare with the predicted graphs.
)
Time (Unit:
)
Total Mechanical Energy (Unit:
)
Time (Unit:
Time (Unit:
)
Measuring g
Based on the acceleration obtained from the Velocity graph, find the value of g.
50
Effect of friction
What is the static friction when the cart on the ramp is not moving? Provide an analysis to
support your answer.
In the case of a cart remain stationary on the ramp, does the static friction depend on how
hard you press the friction block?
Kinetic Energy
Potential Energy
Draw a conclusion from the predicted and measured energies vs time graphs.
Time (s)
Mechanical Energy
Time (s)
Time (s)
51
Paste the screenshot of the position and velocity graphs of cart motion with friction.
Record position and velocity readings in five different times on the graphs of cart motion
with friction and complete the following table.
Table II
Time
Position
𝑥
Height
ℎ = 𝑥𝑠𝑖𝑛𝜃
Potential
Energy
=𝑚𝑔ℎ
1
2
3
4
5
52
Velocity
𝑣
Kinetic
Energy
1
= 𝑚𝑣 2
2
Total
Energy
= 𝑃𝐸 + 𝐾𝐸
Potential Energy (Unit:
Kinetic Energy (Unit:
)
)
Plot the measured energy-time relations with friction using Table II
)
Time (Unit:
)
Total Mechanical Energy (Unit:
)
Time (Unit:
Time (Unit:
)
Use the fact that the change in the mechanical energy is equal to the work done by friction
over a distance to find the value of the friction force acting on the cart.
Use the electronic balance to find the mass of the friction block, and then find the
coefficient of friction between the friction block and the track.
53
Laboratory Exercise #7
Collisions and Momentum
Objectives: To study the relation between force and change in momentum and to
determine whether momentum is conserved in inelastic collisions
Equipment:
Two sensor carts with bumpers, yellow cart with a bumper, electronic
balance, masses, track with end stops, three 125 g masses.
Introduction
Instead of writing Newton’s Second Law as F=ma, we can also write it as 𝐹 =
∆𝑝
.
∆𝑡
This
means that if a constant force is acting on an object for a time t, then its momentum will
change by an amount Ft, called impulse. Let us assume that the force starts acting at a
time t0. If we plot the force as a function of time, it will be zero until t 0, F between t0 and
t0+t, and then zero again after that. The area under the force versus time plot is just Ft,
which is the change in momentum. If the force is not constant, it is still true that the area
under the force versus time plot will be the change of the momentum of the object.
When two objects collide in the absence of external forces, Newton’s Third Law guarantees
that the total momentum is unchanged. If the two colliding objects are object A and object
B, then when they collide, the force exerted by A on B will have the same magnitude as the
force that B exerts on A, but its direction will be opposite. This means that the change in
momentum of object A will be the opposite of the change in the momentum of object B, so
that the sum of their changes in momentum is zero. Therefore, the total momentum before
the collision is the same as the total momentum after the collision. In terms of equations, if
pA=mAvA is the initial momentum of object A pB=mBvB is the initial momentum of object B,
pA=mAvA is the final momentum of object A, and pB=mBvB is the final momentum of object
B, then
pA+pB=PA+PB .
In this lab, we want to verify Newton’s Third Law, show that the change in momentum is
equal to the area under the force versus time graph, and study momentum conservation in
an inelastic collision. The collision will involve two carts, one initially moving and the other
sitting still, that stick together when they hit. This means that if the initially stationary cart is
object B, then vB=0 and VA=VB.
Procedure
1. Elastics Collision
You should have two cars with built-in force sensors. The bumpers are mounted on the
force sensors. Place them both on the track with the arrows pointing to each other. The
54
arrow of the green cart should point to the end stop.
a. Place the green cart at the end and the yellow cart in the middle of the track. You will
be asked to push the green cart, not too hard, toward the yellow cart so that that they
collide. Before you take data, sketch you predictions for what the force versus time plots
for each cart will look like. Note that the force is positive if it is in the same direction of the
arrow.
b. Open Graphical Analysis software. Click Senor Data Collection to connect the two
sensors whose IDs match the ones on your carts.
When both sensors are connected, click SENSOR CHANNELS, enable both Position and
Force sensors. Do this for both sensors. Then click Done.
Now you will see three graphs on the screen. Use View Options at the upper right corner
to select two graphs.
Click the vertical label of the upper graph to select Velocity G and Velocity Y and deselect
all other items. (G means Green and Y means Yellow)
Click the vertical labs of the lower graph to select Force G and Force Y and deselect all
other items.
Click Force G and Force Y on the lower right corner to zero the force sensors.
Click Position Y and Force Y to reverse the direction. This is done because the arrow on
the yellow cart points to the opposite direction of the green cart which is taken as the positive
direction.
c. Now place the yellow cart in the middle of the track, click COLLECT and gently push
the green cart toward the yellow cart so that that they collide. The force sensor will measure
the forces on each of the carts. Catch the cart before they strike the end stop.
Use magnifiers to zoom in and then take a screenshot using your mobile phone.
What does the Force-Time graph tell you about Newton’s Third Law?
d. We now want to see if the total momentum is conserved before and after collision. From
the graph, record the velocity of the green and yellow carts before and after collision.
Next we will test whether the change in the momentum of the initially motionless (yellow)
cart is equal to the applied impulse which is the area under the force versus time plot. Use
the electronic balance to find the mass of the cart that was initially at rest, and then make
use of your velocity-time plot to find change in momentum of this cart due to the collision.
This is just the momentum after the collision minus the momentum before the collision.
Now use the View Integral function under Graph Options at the lower left corner of the
Force-Time graph to find the area under the force curve. Compare the change in momentum
to the area under the force-time graph.
55
e. Next we will repeat the same experiment except that a 375 g is loaded on the stationary
yellow cart. Before you take data, sketch you predictions for what the velocity versus time
plots for each cart will look like.
Then repeat Steps c and d with the yellow cart carrying 375 g (3 x 125g) masses.
2. Inelastic collision:
Now rearrange the empty carts so that the two ends with Velco pads face each other.
You want to have the yellow cart initially moving and the green cart initially at rest in the
middle of the track. The collision will cause the two carts to stick together when they hit
(again, not too hard!).
You can follow the step outlined in Step 1c to obtain velocity data and thereby find the
momentum before and after the collision. Now do the experiment and compare the initial
and final momentum.
56
Laboratory Exercise #7
Collisions and Momentum
Date ______________
NAME:______________________________Partners:________________________
Instructor's Signature:____________________
Collison between two cart of equal masses
Sketch predictions for what the force versus time plots for each cart will look like during
collision.
Yellow cart
Force on Cart 2
Force on Cart 1
Green Cart
Time (s)
Paste the screenshot of the force and velocity graphs during the colllision
What does your Force-Time graph tell you about Newton’s Third Law?
57
Time (s)
Velocity Green Cart
Before collision________________. After collison=__________________.
Velocity of Yellow Cart
Before collision________________. After collison=__________________.
Compare the total momentum of the green and yellow cart before and after collision.
Present your analysis.
Compare the momentum gained by the yellow cart after the collision with the area under
the Force-Time graph.
58
Collison with a mass on the stationary cart
Sketch predictions for what the velocity versus time plots for each cart will look like
during collision when a 375g of mass is loaded on the yellow cart.
Velocity (m/s)
Yellow
Velocity (m/s)
Green
Time (s)
Time (s)
Paste the screenshot of the force and velocity graphs for the collision with a 375 g mass
on the yellow cart during the colllision
Velocity of Green Cart
Before collision________________. After collision=__________________.
Mass of Green Cart_____________
Velocity of Yellow Cart
Before collision________________. After collision=__________________.
Mass of Yellow Cart______________
59
Compare the total momentum of the green and yellow cart before and after collision.
Present your analysis.
Compare the momentum gained by the yellow cart after the collision with the area under
the Force-Time graph.
60
Inelastic Collision
Sketch a prediction for the velocity plots for the two carts before and after collision.
Velocity (m/s)
Green Cart
Velocity (m/s)
Yellow Cart
Time (s)
Time (s)
Past the screenshot
Velocity Yellow Cart
Before collision________________. After collision=__________________.
Velocity of Green Cart
Before collision________________. After collision=__________________.
Compare the total momentum before and after collision.
Present your analysis.
61
LABORATORY EXERCISE # 8
Part I: Simple Harmonic Motion
Objectives
To study the force law for a spring (Hook's Law). To verify the equation for the period of a
vibrating spring.
Equipment and Supplies
Spring, support for suspending spring, set of weights, stop clock, meter stick, balance.
(a) Unstretched spring
(b) Stretched spring
M
M
(c) Force diagram
T= k x
Mg
62
x
Discussion
When a stretching force F is applied to a spring, the elongation of the spring x is found
to be proportional to F if the elastic limit is not exceeded. The force is a restoring force,
i.e., always opposite to the displacement. This empirical law is called Hook's law:
F kx
where k is called the force constant, or the stiffness of the spring. The general equation
of motion for the mass M with acceleration a which is vertically hung from the spring as
seen in the figure is
Ma Mg kx
within the elastic limit. Here, we completely neglect the mass of the spring which in our
experiment is much smaller than the mass attached.
(1) If the mass M is in an equilibrium state, the equation is reduced to
Mg kx
Force F (N)
since a 0 . The elongation x of the spring can be measured as a function of the weight
hung on it when varying the mass M of the suspended weight F Mg and a plot of F vs
x is a straight line, as illustrated below.
Elongation x (m)
The slope of the straight line is the stiffness k of the spring. In the MKS units it is N / m .
(2) If a mass, M, hung from the end of an elastic spring is pulled down and released, it will
oscillate up and down. This is an example of simple harmonic motion. The acceleration a
63
must not be neglected since the mass is not in equilibrium. In this case the equation of
motion is rewritten as
x
k
xg
M
where x a and the displacement at time t is
x
g
A cos(t )
2
k
depends on the mass M and the stiffness of the spring. If the time t is
M
replaced by t+2 / , then the displacement x is unchanged. This means that the
displacement has the same value after time T,
where
T
2
2
M
.
k
This is the period of the oscillation. A is the amplitude of the oscillation and is determined
by the initial displacement of the mass.
Actually, since all parts of the spring also execute simple harmonic motion, the mass of the
spring needs to be included in some way. Since not all parts of the spring execute the full
motion, it turns out that m', the "effective mass" of the spring is just 1/3 of the full inertial
mass, m. So, the parameter M should be the sum of the mass of the hung weight, M0, plus
1/3 of the mass of the spring:
M = M0 + m' = M0 + m/3
where,
M0 is the mass of the suspended weight,
m is the mass of the spring, and
m' is the effective mass of the spring ( m' = m/3).
Procedure
1. F vs. x data.
Measure the elongation x of the spring as a function of the weight hung on it. Vary the mass
of the suspended weight from 200 grams to 800 grams in 100 g steps. Take the position
when 200 g is suspended to be zero displacement ( the origin).
Do not exceed 800 g.
2. T vs. M data.
Hang a mass of 200 grams from the spring and make at least three determinations with a
stop clock of the time for 50 complete oscillations of the mass. Use a different initial
amplitude in each determination. Repeat with masses of 350, 550 and 700 grams.
64
Calculations and Conclusions
A. From the data in Procedure 1, calculate analytically and graphically the force constant
of the spring and compare the two values. The analytic calculations involve using Hook’s
law for each of your data points. The graphical method involves plotting F vs. x, and
measuring the slope of the best straight line through the data points (see the sketch above).
B. Plot T2 vs. M, i.e., the square of the period on the vertical axis and the mass of the
suspended weight on the horizontal axis. From the graph, calculate the value of the
stiffness k.
C. Compare the values of k found in A and B. This means, by what percent do they differ?
Now, in summary, considering both measurements, what is the precision (expressed as a
percent) of your measurement of k?
D. Does the period of simple harmonic motion depend on the amplitude? How well does
your data justify your answer?
65
LABORATORY EXERCISE # 8 Part I
Data Sheet: Simple Harmonic Motion
Date: _________
Name:______________________________ Partners:______________________
Instructor's Signature: ________________________
Mass of spring: ________________
M0
Suspended Mass
(kg)
.200
.300
F= M0 g
Force
(N)
Take as 0
(.300-0.200) x 9.80 =
.400
.500
.600
.700
.800
66
x
Elongation of the spring
(m)
Take as 0
Hung mass:
200 g
350 g
550 g
Time for 50
cycles (sec)
amplitude #1
Time for 50
cycles
(sec)
amplitude #2
Time for 50
cycles (sec)
amplitude #3
Average
of
three
above
times (sec)
Period, T
(sec)
T2 (sec2)
67
700 g
LABORATORY EXERCISE # 8
Part II: The Simple Pendulum
Objective
To study how the period of a simple pendulum depends on its length. To measure the
acceleration due to gravity in the laboratory room.
Equipment and Supplies
Metal sphere suspended by a fine string, stop clock, meter stick, vernier calipers.
Discussion
A simple pendulum is one where all the mass is concentrated at a small "bob". A good
approximation is made by using a massive weight held with a light string from a sturdy
support. If the motion is restricted to "small angles", the motion is closely simple harmonic.
T, the period of the motion, depends only on the length of the string, and the acceleration
of gravity. Surprisingly, the period does not depend on the value of the suspended mass:
T 2
L
g
(1)
where, T is the period of oscillation.
L is the distance from the support point to the center of the massive bob, and
g is the local acceleration due to gravity.
Procedure
1. T vs. L data.
Adjust the pendulum so that L is about 100 cm. Measure L using the meter stick and
vernier calipers. Determine the time for 50 oscillations with the amplitude of motion less
than 10 °.
2. Repeat Procedure 1. using a length approximately 30 cm, and again with L about 60 cm.
Calculations and Conclusions
A. Plot your data as T2 vs. L. The theory, given by Eq. (1), predicts that your data points
g. Thus by measuring the slope of the best straight
line fit, you can pull out the value of g in the lab. What is the value of g you obtain, and what
is the precision you claim for your measurement ( expressed as a percent) ?
B. You have just finished measuring g, the acceleration due to local gravity. Express your
68
result as in the form wxy ±z %. In exercise #2, several weeks ago, you also measured g,
using an entirely different method. For the two results summarize the following information:
a) claimed precision
b) agreement with accepted value
Do the two results agree? The key here is the criterion you used to answer the question.
Add any further relevant comments.
69
LABORATORY EXERCISE # 8 Part II
Data Sheet: Simple Pendulum
Date ______________
Name:______________________________ Partners:__________________________
Instructor's Signature:___________________________
Nominal length
Of pendulum, L
100 cm
60 cm
Actual length, L
Time for
50 oscillations
(sec)
Period, T
(sec)
T2
(sec2 )
70
30 cm
71
LABORATORY EXERCISE # 9
The Sonometer: Vibrations of a Taut String
INTRODUCTION
When you pluck a taut fixed string of length L, the resultant vibrations are a superposition
of many simpler "standing wave" patterns, depicted below:
Note that, in general, n = 2L / n, and fn = nf1, meaning that all harmonics are an integer
multiple of a fundamental frequency, f1. Also note, that the place on a standing wave
where there is no vibration is called a node, and the place where there is maximum
amplitude is called an antinode.
PURPOSE
In this exercise you will excite and study the first few vibrational modes of the taut string.
You will use a sonometer, a single-string "musical" instrument. See figure below.
72
EQUIPMENT AND SUPPLIES
1. Sonometer from Pasco Scientific Company. This instrument allows a known tension to
be placed on a guitar string whose vibrating length can be varied between movable
"bridges."
2. A function generator. It can "drive" an
electromagnet with AC current in the
lower audio range. Note: The guitar
string will be attracted to the AC
electromagnet twice each AC cycle,
because both the North and South poles
are equally good at attracting the steel
string.
3. "Detector" coils capable of picking up
the AC motion of a steel string.
4. A dual-trace oscilloscope to display
both the driving frequency and the
detected string vibrational frequency.
PROCEDURE
A1. Set up the sonometer system. Set the bridges 60 cm apart. Hang a 2 kg mass from
the tensioning lever. Adjust the string tensioning knob so the tensioning lever is horizontal.
A2. Position the driver coil about 5 cm from one of the bridges and the detector about 35
cm away from the same bridge.
A3. Gently pluck the string with a fingertip. The detector coil will pick up an induced voltage
which you can see displayed on the oscilloscope. You can excite different waveforms by
plucking in different places or with a different technique.
Play with this a while. You are seeing a different mix of excited harmonics (another name
for the different modes corresponding to different values of n.)
73
Note: If the oscilloscope does not display, adjust the TRIGGERING: all three levers in the
upper right corner should be flipped up, and the level knob pulled out (auto mode.) Also,
adjust the trace position knobs as necessary.
A4. Set the signal generator to produce a sine wave and set the gain of the oscilloscope of
channel B to 5 mV/cm.
A5. With the function generator amplitude set at about 12 o'clock slowly increase the
frequency of the signal to the driver coils, starting from about 5 Hz. (This part will require a
gentle hand and some patience.)
Listen for an increase in sound from the sonometer and/or an increase in size of the detector
signal on the oscilloscope screen. Frequencies that result in maximum string vibrations are
the resonant frequencies. Determine the lowest frequency at which resonance occurs. This
is the first or fundamental mode (n = 1). Measure this frequency and record it on the data
sheet provided. Note: Because of the effect noted in the equipment section, the resonant
frequency is twice the driving frequency.
Note: For the n = 1 mode, you should actually hear the sonometer hum and produce
enough amplitude vibration to clearly see the central antinode and the nodes at the bridges.
A6. Continue increasing the frequency to find successive resonant frequencies for each
mode - at least five or six. For each resonant mode you find, locate all the nodes, and
record the distance between adjacent nodes. There are two ways to locate notes:
1. Use the "detector" provided as follows: Start with the detector as close as you can to the
free bridge. Watch the oscilloscope display as you slide the detector slowly along the
vibrating string. When you reach a node, the amplitude on the scope will drop to a minimum.
(This method breaks down when you get too close to the "driver" and start to pick up its
field directly.)
2. Use your fingers. Watch the oscilloscope display as you slide your fleshy fingertips along
74
the vibrating string. Even a light touch will kill the vibrations everywhere except when you
touch a node, where there is no vibration to kill, in any case. (Make sure that the detector
is not accidentally left under a node, or this won't work.)
A7. From your results, determine and record the wavelength of each resonant mode you
find. Make use of the fact that the wavelength is twice the distance between adjacent nodes.
B1. Repeat the experimental procedure A, for a string length of 50 cm, obtained by moving
one or both of the bridges.
Compute the average value, and quote the uncertainty of your measurement as a percent.
75
LABORATORY EXERCISE # 9
Data Sheet :Sonometer
Date ______________
Name:______________________________ Partners:_______________________
Instructor's Signature:__________________________
1. What mathematical relationship holds, for a given value of n, between the length L and
Procedure A: String length, L = __________
n= 1
Driver frequency =__________
Actual string frequency f (twice driver frequency) = __________
Distance between nodes =__________
n= 2
Driver frequency =__________
Actual string frequency f2 (twice driver frequency) = __________
Distance between nodes =__________
n= 3
Driver frequency =__________
Actual string frequency f3 (twice driver frequency) = __________
Distance between nodes: __________
__________
Average distance between nodes =__________
n= 4
wa
Driver frequency =__________
Actual string frequency f4 (twice driver frequency) = __________
Distance between nodes: __________
__________
Average distance between nodes =__________
76
wavelength =
__________
n= 5
Driver frequency =__________
Actual string frequency f5 (twice driver frequency) = __________
Distance between nodes: __________
__________ __________ ________
Average distance between nodes =__________
n= 6
wavelength
Driver frequency =__________
Actual string frequency f6 (twice driver frequency) = __________
Distance between nodes: __________
__________ __________ __________
Average Distance between nodes =__________
n= 7
wavelength
Driver frequency =__________
Actual string frequency f7 (twice driver frequency) = __________
Distance between nodes: __________
__________ __________ ________
Average distance between nodes =__________
waveleng
2a. Plot your values of the resonant frequencies versus n, the mode number. On the same
graph, draw the best straight-line fit that goes through the origin. This line is the theoretical
result,
fn = n f1
2b. The slope of this best straight-line fit to the data (which passes through the origin) is
just the fundamental frequency (f1) abstracted from all of your data, not just your first
measurement. How good is the agreement?
Procedure B: Use your own data sheet for this part. Take all necessary data, and present
data and calculations in neat tabular form.
3. To what extent do your results in Procedure B verify the relationship given in the answer
to question 1?
4. For each mode you identified and recorded in the data sheets, compute the wave speed.
77
LABORATORY EXERCISE # 10
Properties of Sound Waves: Speed and Standing Waves
To prepare for this lab please familiarize yourself with the following: speed of
waves, standing waves and resonance.
These topics can be easily located at the website “The Physics Classroom”
Anyone observing a trombone player recognizes immediately that the musician
changes the pitch by altering the length of the convoluted tube ( If you haven’t,
check out this Youtube link https://www.youtube.com/watch?v=ZxODzxY6AvI)
In physics parlance what is being done is that the musician is adjusting the
standing wave within the trombone by altering the length of the resonance
chamber. This in turn alters the wavelength and frequency of the sound wave. Our
ears detect the change in frequency as a change in pitch.
In this lab we will be using our ears to detect the resonant frequency of an air
chamber and by doing so, determine the wavelength of the sound wave. We can
then easily calculate the speed of sound in air. We are also going to see how
cooling the air (changing the property of the medium) affects the speed of sound.
Since you are working with liquid be prepared to do immediate cleanup of
surfaces after any spills.
A
Determine the Speed of Sound In a Tube
Apparatus:
4.5 cm wide PVC tube,
Plastic 2-liter graduated cylinder,
Striker,
3 tuning forks 256 Hz,512 Hz.
and a tuning fork of an unknown frequency,
red and blue dyes,
water that is at both refrigerated/ cooled and at room temperature.
DO NOT STRIKE THE TUNING FORKS ON ANY SURFACE!!! Strike the tuning
forks with the mallet.
Your lab instructor will demonstrate. First fill the graduated cylinder to 800 ml
mark. PUT 3 DROPS OF RED DYE INTO THE WATER AND GENTLY SWIRL IT
AROUND. This improves visual acuity. Insert the PVC tube making sure the water
level reaches just below the top.
78
PLACE THE GRADUATED CYLINDER WITH THE TUBE INSIDE ON THE
CHAIR, NOT ON THE TABLE. This improves the ergonomics of the procedure.
Practice lifting the tube partially out of the water and holding it for 15 seconds.
Then return it into the cylinder.
The set up should look something like this:
Take the 256 Hz tuning fork and strike it with the striker. Practice it a few times.
When you are comfortable with this, strike it one more time. While holding the fork
with one hand, raise the tube out of the water with the other hand. Bring the
tuning fork over the tube, holding the fork horizontally over the tube. SLOWLY
raise or lower the tube. Carefully listen for a sudden change in the amplitude of the
sound. Do not be afraid to put your ear as close as possible to the pipe. When the
amplitude increases and decreases sharply, stop. While holding the tube at this
height, your lab partner should measure the length “L” of the air chamber that is
created.
Length L is measured from the top of the top of the tube to the surface of the
water. Play with this a bit to see how varying the length of the air chamber affects
the amplitude of the sound. Practice this a number of times so that you feel
79
comfortable locating the point of maximum amplitude. Once you feel confident
about locating this spot take 5 separate measures of the length and record the
average the data in Table 1.
Here, the air chamber has one open end at the top and a closed end ( the water)
at the bottom. In this case, the wavelength ƛ is four times the length L. Reading
the text will explain why this is so. Note that there is a small correction factor
included in the equation to account for the magnitude of the inner diameter “d” of
the tube. of the air chamber. Remember that d is in meters not centimeters. Now
calculate the wavelength.
= 4 x ( L +0.3 d)
Enter that value in the space provided in Table 2. Now calculate the speed of the
sound wave for both the 256Hz and 512Hz conditions using the formula below.
Remember to convert your length measured in centimeters to meters.
V= x f
Trial
250Hz
cm.
500Hz
cm.
Unknown
cm.
1
2
3
4
5
L Avg.
Table 1
256Hz
512 Hz
funknown =
Hz
L avg m
ƛ
m.
V m/s
Vavg=
80
Table 2
Now that you have made a determination of the speed of sound, see if your
results are precise. Take the tuning fork with an unknown value and repeat the
previous procedures for determining the wavelength of the unknown tuning fork..
Divide the wavelength value of the unknown frequency into the previously
obtained average speed to obtain the frequency of the unknown tuning fork.
funknown = Vavg /
Once everyone in the class has completed this procedure, your lab instructor will
tell you the actual frequency of the tuning fork.
Calculated frequency of the unknown tuning
fork__________________________Hz.
Actual frequency of the unknown tuning fork
__________________________Hz.
Calculate the % error using the actual value as the accepted value and your
derived value as the experimental value.
% Error freq=_______________________________
B
Speed of Sound and Temperature
The speed of a sound wave also depends on the temperature of the air. You can
compare your calculated value of the speed of sound to its theoretical value based
on the temperature of the room ( in Celsius)
Room Temperature=______________C. ( The thermometer is located in the front
of the room.)
Compared to zero Celsius, this value will become T.
At zero degrees Celsius in air at ordinary pressure, the speed of sound is
accepted as 331 m/s. Now calculate the speed of sound at the higher room
temperature “ T” use the following formula:
V = 331 + (0.6m/s)T℃
Record the average value of the speed of sound that you obtained from the two
81
known tuning forks below and compare it the temperature dependent value.
VTemp = ____________m/s
VAverage = ____________m/s
% Error_________________
If they are close (within 10%), congratulations. Otherwise, list some factors that
might contribute to significant disparities between your values and theoretical
values. Please don’t just say “human error”.
Factors:
C
Changing the speed of sound by cooling the tube.
Since the speed of sound is dependent on the temperature of the air, changing the
temperature, should change the wavelength of the standing wave.
We are going to see how much the speed of sound changes as the air chamber is
cooled. For this step use only the 256 Hz tuning fork. Now get ready to cool the air
chamber.
Empty the cylinder of the room temperature water you have just used. Go to the
refrigerator and take out one bottle of cooled water. Notice that the cold water is
tinted blue with a food dye. Pour cold water from the cold water bottle to the 800
ml mark. Place the tube in the water and give it a moment to cool. Prepare to
measure and record air chamber lengths as you did before. Since the tube will be
colder, it will cool the air inside as well. To anticipate the warming of the air
chamber as the tube is lifted, have the tuning fork and meter stick positioned for
rapid measurement.
Lift the tube as before and strike the tuning fork. As before, hold it horizontally over
the tube as you raise it. When you detect the resonant sound, measure the length
of the air chamber. Lower the tube back into the cold water.
Do this for five trials and record the data. Then determine the average value of the
length.
Trial
1
2
3
Length cm
82
4
5
Length Average
________________cm = _____________m
Calculate the wavelength using the equation:
ƛ = 4 x ( L +0.3 d)
Calculate the experimental speed of sound from
Vexp = ƛ x f
Now using data from the 256 Hz tuning fork, compare the calculated speed of
sound in cool air to the speed of sound obtained in warm room temperature air.
Vwarm = _____________m/s
Vcool = __________________m/s
Theoretically, the speed of sound changes by 0.6 meters per second per degree
Celsius. While it is not feasible to actually measure the cooler temperature inside
the chamber, we can get an estimate of how cold the air inside the chamber is.
Using your value of Vcool and the equation Vcool = 331 + (0.6m/s)𝚫T℃,
determine the temperature for your chamber. On the board, let the class list the
temperature calculated by each table. Discard the highest and lowest values and
find the average of the remaining values. Show your work.
Air Chamber Temperature Class Average ________________Celsius
1. Investigate how the speed of sound is directly proportional to the
temperature of the air chamber. Be brief.
2. Investigate how the speed of sound in air changes with changes in
atmospheric pressure.
83
3. Describe two other instruments that alter their resonant frequency by
adjusting the length of the air chamber.
84
LABORATORY EXERCISE # 11
The Mechanical Equivalent of Heat
Objective
The purpose of this Laboratory Exercise is to prove the mechanical equivalence of heat
and to verify that 1 kcal = 4186 Joules.
Equipment and Supplies
Commercial apparatus is provided which consists of the following:
1. An aluminum cylinder whose specific heat you know, whose mass you will soon measure,
and whose temperature is conveniently sensed by a built-in thermistor. [A thermistor is a
resistor whose resistance varies with temperature]. You are provided with a digital
ohmmeter and a Table to tell you what temperature corresponds to what resistance. This
often requires interpolation. If you are rusty on interpolation, ask your instructor.
2. A hand-crank and digital counter to record the number of revolutions.
3. A nylon rope with a "10 kg" weight at one end. The rope is wrapped a few times around
the aluminum cylinder so that tangential frictional forces are set up between rope and
aluminum cylinder when you turn the crank. CAUTION: Allowing the 10 kg weight to rise
more than a few inches off the floor is dangerous both to student feet and to the delicate
one-way digital counter.
CAUTION:
The Proper Procedure for turning and stopping the crank will be
demonstrated by your instructor. It may look simple enough, but in fact it is easy to
hurt yourself or damage the equipment. Respect this warning!
Discussion
Mechanical Work, which for linear motion is simply "force times distance", takes on the
following expression for circular motion:
W = (Torque) x angle,
W = (Tangential force x Radius) x angle,
W = F · R · θ , where θ is in radians.
For example, a force of 2.00 N acting for one complete revolution (2π radians) around a
circle of radius 1.00 meter does an amount of work given by,
W =F · R · θ = (2.00 N)(1.00 m)(2π radians) = 4π J = 12.6 Joules.
The heat energy Q transferred to a body of mass m and heat capacity c, will raise its
temperature by an amount ΔT degrees, where
85
Q = mcΔT
For example, if the temperature of 0.10 kg of Aluminum (c = 0.220 kcal/kg °C) is seen to
rise from 21.5 °C to 38.5 °C, we can conclude that an amount of heat Q must have been
added to the aluminum, where
Q = m cΔT = (0.10 kg)(0.220 kcal/kg °C)(38.5 °C - 21.5 °C) = 0.38 kcal.
Procedure
A. Introduction:
A1. Familiarize yourself with the apparatus. Find all the parts in the diagram below.
A2.
Note
how
sensitive the thermistor resistance is to the touch of your hot little hands, and how quickly
or slowly thermal equilibrium is approached. Now you know the timescale of the response
of this equipment.
A3. When confident, unscrew the black knob that holds the aluminum cylinder in place and
weigh the cylinder. Measure its diameter alone, and the diameter of the cylinder plus rope,
when tightly wound.
A4. Weigh the "10 kg" mass to 3 significant figures.
86
B. "Quick and Dirty" Run
B1. Note the approximate initial temperature of the aluminum cylinder. Look-up what thermistor
resistance corresponds to a "target" final temperature about 8 Celsius degrees higher. Your aim is
to do enough mechanical work to heat up the aluminum cylinder to the target temperature.
B2. Reset the counter. Enter the actual initial temperature in your data sheet and start cranking
vigorously! Now you are finally getting "physical" in your physics class!
B3. When you get to your target temperature, stop cranking BUT DO NOT LET GO OF THE
CRANK. Allow the "10 kg" weight to drift to the floor before slowly returning the crank to its
equilibrium position. Do not let go prematurely or the crank will snap back, possibly damaging the
plastic clicker and invaluable body parts.
Continue to watch the thermistor resistance carefully and enter in your data sheet the lowest value
(highest temperature) it drifts to.
B4. Record the total number of turns and complete the calculations called for in your data sheet.
C. Serious Run
Even when done by an expert student, one of the major sources of error in Procedure B is that some
of the heat generated will go to heat up the surroundings rather than the aluminum cylinder. To
counteract this, in this procedure you will precool the cylinder so that during your run the temperature
of the block will be as much below room temperature as above it. Then there should be as much
heat gained from the surroundings as lost to them. It is by tricks of this nature that successful
experiments are accomplished.
C1. Note the temperature of the room. Plan your target initial and final temperatures to be about 8
°C below and above room temperature, respectively.
C2. Remove the aluminum cylinder and cool it in the ice chest provided. Use plastic baggies, if
available, to prevent wetting. You may not need to cool it all the way to 0 °C. In any event, the heat
from your hands can now be skillfully used to make-up for too much precooling.
NOTE: Carefully dry any condensed water from the aluminum cylinder with the tissue paper
provided. Do this before re-wrapping the friction rope. Evaporating water could carry away a
considerable amount of heat; heat that would otherwise go to warm up the aluminum.
Now, re-assemble the apparatus, reset the counter and get ready. Watch the ohmmeter carefully
as the thermistor resistance drifts toward your predesignated "target" for the initial temperature.
When it gets there, start cranking vigorously.
Crank until the temperature reaches approximately one degree short of your designated final
temperature. Then crank slowly until you reach your target. Record the actual maximum
temperature (minimum resistance) reached. Record the total number of turns. REMEMBER THE
SAFE STOPPING PROCEDURE. A MISTAKE CAN RESULT IN YOUR HURTING YOURSELF.
C3. Compute the work done, the heat input, and the mechanical equivalent of heat as guided by
your data sheet. Compute the percent deviation from the accepted value.
87
D. Consider the possible reasons (mechanisms) why your answer is different from the accepted
value. Do you have any suggestions to improve this experiment?
Resistance versus Temperature for your thermistor:
Res.
(Ω)
T
(°C)
351,020 0
332,640 1
315,320 2
298,990 3
283,600 4
269,080 5
255,380 6
242,460 7
230,260 8
218,730 9
207,850 10
197,560 11
187,840 12
178,650 13
169,950 14
161,730 15
153,950 16
146,580 17
139,610 18
133,000 19
126,740 20
120,810 21
115,190 22
109,850 23
104,800 24
100,000 25
Res.
(Ω )
T
(°C)
95,447 26
91,126 27
87,022 28
83,124 29
79,422 30
75,903 3
72,560 32
69,380 33
66,356 34
63,480 35
60,743 36
58,138 37
55,658 38
53,297 39
51,048 40
48,905 41
46,863 42
44,917 43
43,062 44
41,292 45
39,605 46
37,995 47
36,458 48
34,991 49
33,591 50
32,253 51
Res.
(Ω )
T
(°C)
30,976 52
29,756 53
28,590 54
27,475 55
26,409 56
25,390 57
24,415 58
23,483 59
22,590 60
21,736 61
20,919 62
20,136 63
19,386 64
18,668 65
17,980 66
17,321 67
16,689 68
16,083 69
15,502 70
14,945 71
14,410 72
13,897 73
13,405 74
12,932 75
12,479 76
12,043 77
88
Res.
(Ω )
T
(°C)
11,625 78
11,223 79
10,837 80
10,467 81
10,110 82
9,767.2 83
9,437.7 84
9,120.8 85
8,816.0 86
8,522.7 87
8,240.6 88
7,969.1 89
7,707.7 90
7,456.2 91
7,214.0 92
6,980.6 93
6,755.9 94
6,539.4 95
6,330.8 96
6,129.8 97
5,936.1 98
5,749.3 99
LABORATORY EXERCISE # 11
DATA SHEET :
Mechanical Equiv. of Heat
Date:_____________
Name:_________________________________ Partners:_____________________
Instructor's Signature:__________________
A. Fixed parameters
Specific heat of Aluminum, c = 0.220 kcal/kg °C
Mass of Aluminum cylinder, m = ___
Diameter of cylinder, D1=_________
Diameter of cylinder plus rope, D2=_______
Average, Do = ( D1 + D2)/2=_________
Average Radius, R = Do/2=_________
Mass of "10 kg" mass, to 3 significant figures, m=__________kg
Weight of "10 kg" mass, to 3 significant figures, F=__________Newtons
B. "Quick and Dirty" Run
B1. Approximate initial temperature:
Thermistor Resistance =_________
Corresponding Temperature = __________
"Target" final temperature:
Target Temperature =_________
Thermistor Resistance=__________
B2. Actual starting temperature:
Thermistor Resistance =__________
Corresponding Temperature =________
B3. Actual final temp.:
Thermistor Resistance =__________
Corresponding Temperature =___________
B4. Number of turns, N =__________
Total angle, θ
B5. Computed total work you did, W = F R θ =___________Joules
Computed heat input to Aluminum cylinder, Q = mcΔT = ___________kcal
Your computed Mechanical Equivalent of Heat,
(ratio of above two results):_______________
89
What is the percent deviation of your result from the accepted value of 4186 J/kcal?
____________ %
C1. Room Temperature today____________ °C
Target initial temperature____________ °C
Corresponding Thermistor Resistance__________Ω
Target final temperature_____________ °C
Corresponding Thermistor Resistance____________Ω
C2. Actual initial temp.:
Thermistor Resistance =__________ Corresponding Temperature = ___________ °C
Actual final temp:
Thermistor Resistance =__________ Corresponding Temperature = ___________ °C
Number of Turns__________Total angle, θ
C3. Compute the total work you did against friction:
W = FRθ = ___________Joules
Compute the amount of heat that must have been transferred to the Aluminum,
Q = mcΔT = ____________kcal
Compute the Mechanical Equivalent of Heat and the percent deviation:
D. Did you do better in Part C than in Part B? If not, what went wrong? Give reasons.
90
LABORATORY EXERCISE # 12
Boyle's Law
Objectives
To study how changing the pressure on a gas makes the volume change. To compare your
results with those obtained by Bobby Boyle.
Equipment and Supplies
A sample of air trapped at the bottom of a glass tube by a short column of mercury.
A ruler to measure the length of the air column.
A barometer somewhere in the room.
Discussion
According to Robert Boyle, if the temperature of a fixed amount of gas is not allowed to
change, the following relationship will hold as the volume, V, and the pressure, P, are
changed:
P · V = a constant number
(1)
The value of the constant depends on the number of moles of gas, the temperature, and
the particular units used to measure Pressure and Volume. Boyle's Law accurately
describes the constancy of the pressure-volume product for most common gases at
moderate temperatures and pressures. Deviations from this Law may become significant
if the pressure is too high or the temperature is too low. These limiting values vary
drastically with different gases. For example, hydrogen obeys Boyle's Law at -200 °C and
100 atmospheres; sulfur dioxide does not at 20 °C and 1 atmosphere.
The apparatus for this experiment is elegant in its simplicity. The short mercury column in
the glass capillary serves two purposes. First, it serves as a leak-proof piston to isolate
your gas sample. Second, it provides additional pressure on the gas sample as the glass
tube is inclined. By varying the vertical orientation of the glass tube, you can vary the
pressure of the gas sample. To see how this works, note that the absolute pressure of the
enclosed air in Figure (1a) is,
P = Patm + ρ g (Y1 - Y2)
(2)
where Patm
= atmospheric pressure,
ρ
= the density of mercury, 13,000 kg/m3, and
Y1 and Y2
= the vertical heights of the ends of the mercury column.
If atmospheric pressure is expressed in centimeters of mercury, call it B cm of Hg, (obtained
directly from the mercury barometer at the end of your lab room) then the absolute pressure
of the gas sample of Fig (1a) can be simply written as,
91
P = ρ g (B + Y1 Y2)
(3)
Life
is
simplest if you express the pressure of the gas in units of cm of Hg instead of N/m 2.
Equation (3) becomes,
P = B + Y1 - Y2
(4)
Now, for one more simplification: Because the cross-sectional area of the capillary is a
constant, the volume of the gas is directly proportional to its length, L. As a result, Boyle's
Law for your gas in the capillary is simply,
( B + Y1 Y2) L = a constant number
(5)
Note: When the open end of the capillary is lower than the closed end, ( Y1 - Y2) is negative.
See Figure (1b). As Equation (4) predicts, in that case the pressure of the gas will be less
than atmospheric pressure. So, the extremes of pressure you can obtain are B + M and B
- M.
Procedure
1. Measure atmospheric pressure in cm of Hg using the classroom barometer.
92
Locate the barometer, examine its parts. Adjust the barometer so that the surface of the
mercury in the cup at the bottom of the instrument just touches the apex of the ivory cone.
This marks the zero point of the barometer scale. Next, align the lower surface of the vernier
slide with the top of the mercury column. (Use the top of the meniscus). Record the mercury
level in your Data Sheet. Some days the pressure can change measurably during the
course of the experiment; take another reading at the end of the lab period and use the
average value if you notice a change.
2. Measure the length of the air column as you vary the pressure of the gas.
For ten positions of the apparatus, chosen to yield approximately equal increments of
pressure, record the length, L, of the air column, and the heights Y1 and Y2. Two of the
positions must correspond to the extremes of pressure. Note: The best way to measure
L is to measure the positions of the endpoints of the air column, L =X1 - X2.
Calculations and Conclusions
1. Plot P vs. L and P · L vs. L on a single sheet of graph paper.
Calculate the product P · L for each value of L you chose, then plot the graphs requested.
Suppress the origin of the abscissa so that the range of L observations covers the horizontal
scale. Do not suppress the origin of the ordinates, otherwise the experimental errors will
be magnified on the graph. Draw a smooth curve through the P vs. L points. Draw the best
straight line through the P · L vs. L points. Which graph is better suited to judge how well
your data follows Boyle's Law?
2. Plot P vs. 1/L.
Draw the best straight-line fit through the origin, representing "the theory", i.e., Boyle's Law.
How well does your data follow the accepted theory? Comment on possible reasons for
disagreement, if there is any in your case.
93
LABORATORY EXERCISE # 12
Boyle's Law: Data
Date ______________
NAME:______________________________Partners:________________________
Instructor's Signature:____________________
Boyle's Law
B
(Air Pressure, cm of H)
Temperature
°C
Trail #!
Trail #2
Average
Length of mercury column, M=______________________________
Trial #
X1
cm
X2
cm
Y1
cm
L=x
cm
94
Y2
cm
Y=
Y1-Y2
P, cm
=B+Y
P xL
cm2
LABORATORY EXERCISE # 13
Part I: Heat of Fusion of Ice
Objective
To determine the heat of fusion of ice by the method of mixtures.
Equipment and Supplies
Calorimeter, balance, thermometer, ice cubes.
Discussion
The change of a substance from the solid to the liquid state, called melting or fusion takes
place at a definite temperature characteristic of each substance. During the transition no
change in temperature occurs. To bring about this change of state a definite amount of
heat must be supplied from the surroundings for each unit mass melted. This quantity of
heat is called the (latent) heat of fusion of the substance.
Thus the amount of heat required to melt a solid of mass m and heat of fusion Hf without
changing its temperature is mHf .
This is also the amount of heat given up to the surroundings when the liquid changes to the
solid state. In the metric system Hf is measured in calories/gram or kilocalories/kilogram.
(Numerically these are the same. )
If two substances at different temperatures are mixed in a container thermally insulated
from its surroundings, the final temperature of the mixture will be between the original
temperatures. From the principle of the conservation of energy, the heat lost by the warmer
substance must equal the heat gained by the colder:
Heat gained by colder body = Heat lost by warmer body
To determine the heat of fusion of water by this method of mixtures, a piece of ice at 0 ° C
is dropped into a calorimeter containing warm water. The initial temperature of the water,
its final temperature after being cooled by the ice and the masses of the ice, warm water
and calorimeter are measured. If the specific heat of the calorimeter is known, the heat of
fusion of water may be determined using the fact that the heat absorbed by the ice in
melting, plus the heat needed to raise the temperature of the resulting cold water to the final
temperature of the mixture equals the heat lost by the warm water and calorimeter:
[ m Hf + m c ΔT ]ICE = [ m' c ΔT '']WARM WATER + [ m" c" ΔT'']CALORIMETER
Here, m, m' and m'' are the masses of the ice cube, the warm water and the calorimeter,
respectively. Also, c and c'' are the specific heats of water and aluminum. Finally, ΔT is
95
the temperature rise of the ice, while ΔT '' is the temperature fall of the warm water.
Procedure
1. Weigh the inner calorimeter vessel and stirrer, fill it approximately two-thirds full of water
about five degrees above room temperature and weigh again to determine the mass of the
water. Place the calorimeter vessel inside the calorimeter and insert a thermometer to
determine the temperature of the water and vessel.
2. Stir the water in the calorimeter well and record its temperature. Dry an ice cube, place
it in the water, replace the calorimeter cover and, when the ice has melted, stir the water
and record its final temperature.
3. Weigh the calorimeter vessel, stirrer and contents to determine the mass of the ice used.
Calculations and Questions
A. Calculate from your data the heat of fusion of water.
B. Compare the value found in A and the generally accepted value for the heat of fusion of
water.
C. Why was it desirable to have the initial temperature of the water slightly above the
temperature of the room?
Note: The data sheet for this experiment and the next are combined into one. You will find
it following the next experiment.
96
LABORATORY EXERCISE # 13
Part II: The Specific Heat of a Metal
Objective
Determine the specific heat of a metal by the method of mixtures.
Equipment and Supplies
Calorimeter, steam boiler, metal shot, balance, two thermometers, small beaker.
Discussion
The "specific heat", c, of a substance is the amount of heat required to raise the temperature
of a unit mass one degree. If the specific heat is independent of the temperature**, the
amount of heat, Q, required to raise the temperature of a substance whose mass is m and
whose specific heat is c, from an initial temperature Ti to a final temperature Tf , is,
Q = m c ( Tf - Ti ) = m c ΔT
Since the calorie (kilocalorie) is defined as the amount of heat required to raise the
temperature of 1 gram (1 kilogram) of water 1 ° Celsius from 14. 5 ° to 15. 5 °, the specific
heat of water is 1 cal/gm °C (1 kilocal/kgm °C).
If two substances at different temperatures are mixed in a container thermally insulated
from its surroundings, the final temperature of the mixture will be between the original
temperatures. From the principle of the conservation of energy, the heat lost by the warmer
substance must equal the heat gained by the colder:
Heat gained by colder body = Heat lost by warmer body.
You will determine the specific heat of a metal by this method of mixtures. A mass of metal
pellets at a high temperature is dropped into a calorimeter containing cold water. You will
measure the initial temperature of the metal pellets and of the water, the final temperature
of the mixture and the masses of the components (metal pellets, water and calorimeter.) If
the specific heat of the calorimeter is known or if the calorimeter is made of the metal whose
specific heat is being determined, the specific heat of the metal may then be determined by
using the fact that the heat lost by the metal must equal the heat gained by the water and
the calorimeter.
**
Specific heats actually vary slightly with temperature. However, for the range of
temperatures encountered in this experiment and for the expected precision, this variation
may be safely neglected.
97
Procedure
1. Heat the water in the boiler. Weigh the boiler cup, fill it approximately two-thirds full of
metal pellets (copper or aluminum) and weigh again to determine the mass of the pellets.
Place the cup in the boiler and insert a thermometer in contact with the pellets.
2. While the metal pellets are heating, weigh the inner calorimeter vessel and stirrer, fill it
approximately two-thirds full of water which is a few degrees below room temperature and
weigh again to determine the mass of the water. Place the calorimeter vessel inside the
calorimeter and insert a thermometer to determine the temperature of the water and vessel.
3. When the temperature of the metal pellets has become stationary at nearly 100 °C, record
its temperature and that of the water in the calorimeter. Be sure that the water has been
stirred well. Then quickly pour the shot into the water being careful that no water is splashed
out. Replace the calorimeter cover, stir well and record the highest temperature to which
the water rises.
4. If the calorimeter is not made of the same metal as the shot, obtain from your instructor
the specific heat of the calorimeter. (The calorimeter is made of aluminum.)
Calculations and Conclusions
A. Calculate from your data the specific heat of the metal used.
B. Compare the value found in A with the generally accepted value for the specific heat of
the metal used.
C. Why was it desirable to have the initial temperature of the water slightly below the
temperature of the room?
98
LABORATORY EXERCISE # 13
Data Sheet for both calorimetry Labs Date ______________
Name:______________________________Partners:_____________________________
Instrutor's Signature:_________________
Calorimetry (1): Heat of Fusion of Water
1.
(a) Mass of calorimeter & stirrer:_________________________________gm
(b) Mass of calorimeter and stirrer 2/3 full of water:__________________gm
{water is ~5 ° above room temperature}
(c) Mass of water (difference of above two masses): _________________gm
2.
(a) Initial temperature of water, calorimeter & stirrer:__________________ °C
{Here, you added towel-dried ice cubes}
(b) Final temperature of water, calorimeter & stirrer and melted ice:______ °C
(c) ΔT , temperature change, from the above two temperatures:_________ °C
3.
(a) Final mass of calorimeter & stirrer, original water plus melted ice:_____ °C
(b) Mass of ice cubes {line 3(a) minus line 1(b)}:_____________________gm
Do not empty or add more water!
All will be used "as is" for the next exercise ( # 14).
99
Calorimetry (2): The Specific Heat of a Metal
4.
(a) Initial mass of boiler cup:____________________________________gm
(b) Mass of cup plus metal pellets:_______________________________gm
(c) Mass of metal pellets {line 1(a) minus line 1(b)}:__________________gm
5.
(a) Mass of calorimeter & stirrer {same as line 1(a) from other side}:_____gm
(b) Mass of calorimeter & stirrer and cool water:_____________________gm
{same as line 3(a) if no water was added or removed}
(c) Mass of water { line 5(b) minus line 5(a)}:_______________________gm
6.
(a) Temperature of hot metal pellets:______________________________ °C
(b) Initial temperature of water and calorimeter & stirrer:_______________ °C
(c) Final highest temperature of mixture:___________________________ °C
7.
(a) Specific heat of calorimeter & stirrer:______________________cal/gm °C
{given by instructor, if needed}
100