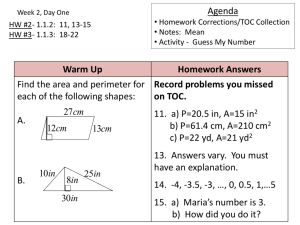
CHEMICAL ENGINEERING DEPARTMENT APPLIED CHEMISTRY LAB (CH10031) 4.Determination of Total Organic Carbon of liquid sample (TOC)s Batch-2 Group-7 Members: 1. Tushar Kumar - Roll Number: CH23BTECH11043 2. Saragadam Lokesh - Roll Number: CH23BTECH11040 3. Sanjay Meena Date: 12/03/2024 - Roll Number: CH23BTECH11038 Table of Contents 1. Aim 2. Apparatus/Equipment 3. Theory 4. Schematic Representation of the Experiment 5. Procedure 6. Observations 7. Calculations 8. Result 9. Discussion AIM: To determine the total organic carbon concentration in given water sample. APPARATUS/EQUIPMENT: 1. TOC-L analyzer 2. Glass beakers 3. Water samples for testing THEORY: TOC, which represents the total amount of carbon present in organic compounds within a sample, is crucial for assessing water quality and environmental cleanliness. In contrast, Inorganic Carbon (IC) is carbon in forms such as carbonates, bicarbonates, and dissolved carbon dioxide. The distinction between organic and inorganic carbon is vital for accurate TOC analysis. The experimental process involves the utilization of a TOC-L analyzer, specifically designed for liquid sample analysis. Prior to analysis, the sample undergoes purging with carbon-free air or nitrogen to eliminate inorganic carbon components. Acid (HCl) is added to the sample inorganic carbon will react with this acid and form 𝐶𝑂2 which is then sparged (a process in which a gas is bubbled through a liquid to remove other gases or volatile compounds) the 𝐶𝑂2 formed in this step can be used to calculate the concentration of inorganic carbon. CaCO3 + 2HCl → CaCl2 + CO2 + H2O Na2CO3 + 2HCl → 2NaCl + CO2+ H2O Purging the inorganic carbon ensures that only organic carbon remains for measurement. During combustion, organic carbon compounds oxidize into carbon dioxide (CO2), which is subsequently quantified using non-dispersive infrared (NDIR) analysis. NDIR: NDIR is an industry term for nondispersive infrared and is the most common type of sensor used to measure carbon dioxide (CO2). The NDIR sensors work by using an infrared (IR) lamp to direct waves of light through a tube filled with a sample of air. This air moves toward an optical filter in front of an IR light detector. The IR light detector measures the amount of IR light that passes through the optical filter. In an NDIR CO2 sensor, the band of IR radiation also produced by the lamp is very close to the 4.26-micron absorption band of CO2. Because the IR spectrum of CO2 is unique, matching the light source wavelength serves as a signature or "fingerprint" to identify the CO2 molecule. As the IR light passes through the sample tube of air, the CO2 gas molecules absorb the specific band of IR light while letting other wavelengths of light pass through. At the detector end, the remaining light hits an optical filter that absorbs every wavelength of light except the wavelength absorbed by CO2 molecules in the sample tube. Finally, an IR detector reads the remaining amount of light that was not absorbed by the CO2 molecules or the optical filter, the calculation for concentration of CO2 is done using Beer-Lambert Law. The Beer-Lambert law is a linear relationship between the absorbance and the concentration, molar absorption coefficient and optical path length of a solution: Another way of calculating TOC is by subtracting the detected inorganic carbon content from the total carbon content. This calculation yields the concentration of TOC in the sample, expressed in various units. To ensure accuracy and reliability, multiple measurements are conducted, and the mean value is typically used to represent the TOC concentration of the sample accurately. Overall, the experiment combines principles of carbon analysis, purification techniques, combustion, and spectroscopic detection to quantify the total organic carbon content in liquid samples. The use of advanced instrumentation and rigorous protocols ensures precise determination of TOC, which is essential for evaluating water quality and assessing environmental pollution levels. SCHEMATIC REPRESENTATION OF THE EXPERIMENT: PROCEDURE: 1. Take a sample of water into a small beaker. 2. Cover the beaker with Paraffin sheet so that the dust particles will not fall into the beaker. 3. Insert the suction tube into the beaker so that it takes water sample from the beaker to the machine to calculate the organic carbon present in it. 4. Switch on the TOC-L analyzer and corresponding desktop app. 5. Fill in the all the required details like the type of sample (POC), amount of sample to be taken for each measurement (20 uL), maximum allowable standard deviation, number of measurements to be taken etc., and start the program. 6. The analyzer will then start calculating the TOC in the sample 7. First it cleans the glass column which holds the specified amount of sample. 8. Then it takes in the specified amount of sample, acidifies it to loosen the bonds and convert carbonates and bicarbonates ions to carbon dioxide. 9. Then it purges the sample with air for 30 seconds to remove all the inorganic content of the sample (CO2) 10. The sample is then transferred to the combustion chamber where it is subjected to combustion and the water vapor is separated by the dehumidifier. 11. The CO2 collected is then subjected to non-dispersive infrared (NDIR) analysis. 12. Finally, the TOC of the sample is calculated based on the results of NDIR analysis. 13. The CO2 produced is absorbed by the scrubber. 14. The mean of three close measurements out of five measured values is taken as the TOC of the sample. 15. Repeat the above procedure with other water samples with three measurements. 16. The mean of two close measurements of TOC is considered as the TOC of the sample. OBSERVATIONS: TOC analysis of Sample1 TOC analysis of Sample 2 TOC analysis of Sample 3 CALCULATIONS: Weight of the injected sample in grams =20uL X Density of sample(gm/L) =20*10-6*103gm =0.02 Organic content in 20uL water in g (wt org) = TOC*10-6 *(weight of the injected sample(gm) Percentage of TOC = (Total organic carbon (gm)/ weight of the injected sample (gm)*100 Percentage of TOC = (wt org/0.02)*100 For sample1: Organic content in 20uL water in g (wt org) = TOC*10-6 *(weight of the injected sample = 1.439 ∗ 10−6 ∗ 0.02 = 2.878 ∗ 10−8 Percentage of TOC = (Total organic carbon (gm)/ weight of the injected sample (gm)*100 = (2.878 ∗ 10−8/0.02) ∗ 100 = 1.44 ∗ 10−4 For sample2: Organic content in 20uL water in g (wt org) = TOC*10-6 *(weight of the injected sample = 36.17 ∗ 10−6 ∗ 0.02 = 7.234 ∗ 10−7 Percentage of TOC = (Total organic carbon (gm)/ weight of the injected sample (gm)*100 = (7.234 ∗ 10−7/0.02) ∗ 100 = 3.617 ∗ 10−3 For sample3: Organic content in 20uL water in g (wt org) = TOC*10-6 *(weight of the injected sample = 38.24 ∗ 10−6 ∗ 0.02 = 7.648 ∗ 10−7 Percentage of TOC = (Total organic carbon (gm)/ weight of the injected sample (gm)*100 = (7.648 ∗ 10−7/0.02) ∗ 100 = 3.824 ∗ 10−3 RESULTS: Following was found after the calculations. S.no Sample Name 1 Sample1 2 Sample2 3 Sample3 Analysis TOC-L TOC-L TOC-L TOC (ppm) 1.439 36.17 38.24 TOC (gms) %TOC 2.878 ∗ 10−8 7.234 ∗ 10−7 7.648 ∗ 10−7 1.44 ∗ 10−4 3.617 ∗ 10−3 3.824 ∗ 10−3 DISCUSSION: 1.How is the Total Carbon Content (TCC) found The total carbon content, which includes both organic and inorganic carbon, is measured by combusting a sample and then detecting the amount of carbon dioxide (CO2) produced. The entire sample is combusted at high temperatures (around 900°C) in an oxygen atmosphere. This process converts all the carbon in the sample to CO2, which is then measured to determine the total carbon content. 2.What is the range of measurement for the CO2 concentration Measurements over the range: TC: 0 to 30,000 mg/L IC: 0 to 3,000 mg/L (Option) POC: 0 to 500 mg/L 3.What happens to CO2 after the experiment After the experiment is done the CO2 evolved is absorbed in soda lime (𝐶𝑎𝐻𝑁𝑎𝑂2 )( soda lime is a mixture of quick lime(CaO) and caustic soda(NaOH) ) 4.How is the Combustion Chamber Maintained at a constant temperature Cooling jackets are channels built into the engine block that surround the combustion chamber. Coolant, either water or oil, circulates through these jackets. As the coolant flows past the hot chamber walls, it absorbs heat, carrying it away from the engine. The coolant then travels to the radiator, where it releases the heat to the surrounding air. Chamber design also plays a role in heat distribution. Different shapes can promote better heat transfer to the coolant. For example, chambers with a larger surface area in contact with the coolant jacket will transfer heat more efficiently. Strategic airflow is another method for controlling temperature. By introducing extra air into the chamber beyond what's needed for combustion, engineers can cool the burning mixture. This cooler air mixes with the hot combustion gases, lowering the overall temperature.