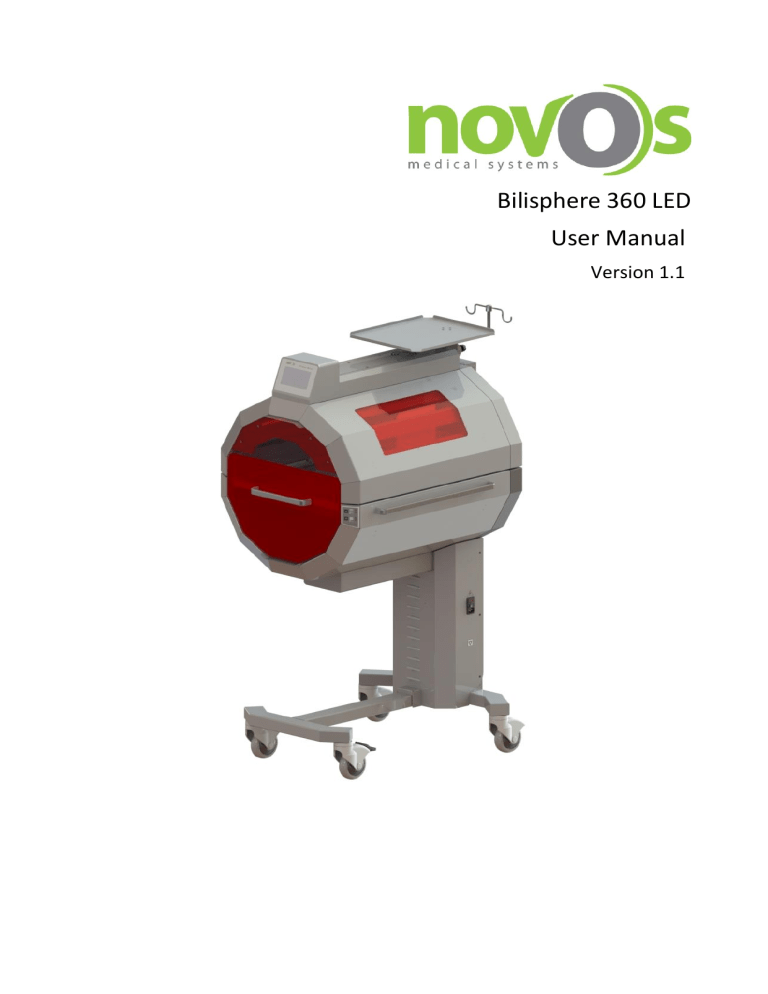
Bilisphere 360 LED User Manual Version 1.1 Revisions Version 1.0 Date of Publish April 2017 1.1 November 2017 Sections Changed Demo mode, Spanish language feature and some minor corrections NOTICE Proprietary Information: This document contains information in which NOVOS, claimed proprietary rights. The information may not be reproduced in whole or in part except as authorized in writing by NOVOS. This information is the property of NOVOS, it is provided solely for use intended. Repairs/Modifications: Repairs on this device shall be performed only by NOVOS or Factory Authorized Service Centers. Information about repairs can be obtained from NOVOS or Authorized Dealers. NOVOS will not be responsible for injury to persons or damage to property arising directly or indirectly from unauthorized repairs or modifications to this device. Furthermore, any unauthorized repairs or modifications void any warranty extended by NOVOS. This document is provided for your information only. It is not changed or updated without request. Definitions: WARNING! Warnings are directions which, if they are not followed, can cause fatal or serious injuries to a user, engineer, patient or any other person or can lead to a mistreatment. CAUTION! Cautions are directions which, if they are not followed, can cause damage to the system described in this manual. Contents 1. Safety Information.................................................................................................................................................................. 1 1.1. Symbols ............................................................................................................................................................................... 1 1.2. Operator's Responsibility for Patient Safety ....................................................................................................................... 2 1.3. Patient Monitoring .............................................................................................................................................................. 2 1.4. Limitation of Liability ........................................................................................................................................................... 2 1.5. Warranty ............................................................................................................................................................................. 3 2. Intended Use .......................................................................................................................................................................... 4 2.1. Applications ......................................................................................................................................................................... 4 2.2. Restrictions of Use ............................................................................................................................................................... 4 3. Parts and Controls .................................................................................................................................................................. 5 3.1. Isometric View ..................................................................................................................................................................... 5 3.2. Front View ........................................................................................................................................................................... 6 3.3. Side View ............................................................................................................................................................................. 7 3.4. Control Panel ....................................................................................................................................................................... 8 3.4.1. Main Screen .................................................................................................................................................................. 8 3.5. Temperature Sensors .......................................................................................................................................................... 9 4. Preparation ........................................................................................................................................................................... 10 4.1. Unpacking and Assembly ................................................................................................................................................... 10 4.2. Electronical Operation Checkout ....................................................................................................................................... 13 5. Operation ............................................................................................................................................................................. 21 5.1. Switching Bilisphere 360 On .............................................................................................................................................. 21 5.2. Control Panel Operation .................................................................................................................................................... 23 5.2.1. Main Screen ................................................................................................................................................................ 23 5.2.2. Alarms and Warnings ................................................................................................................................................. 24 5.2.3. Settings Menu ............................................................................................................................................................ 28 5.3. How to Start a Therapy ...................................................................................................................................................... 33 5.4. Temperature Sensors ........................................................................................................................................................ 38 5.4.1. Attachment/Detachment of Skin Probe ..................................................................................................................... 39 5.5. Infant Bed .......................................................................................................................................................................... 40 5.5.1. Baby Cot ..................................................................................................................................................................... 40 5.5.2. Hammock ................................................................................................................................................................... 41 5.6. Thermo-Elevation .............................................................................................................................................................. 42 5.6.1. Operating Principle ..................................................................................................................................................... 42 5.7. Manual Upper Couple Height Adjustment ........................................................................................................................ 45 6. Routine Cleaning and Maintenance ..................................................................................................................................... 46 6.1. General Cleaning ............................................................................................................................................................... 46 6.2. Maintenance ..................................................................................................................................................................... 47 6.2.1. Lamp Replacement ..................................................................................................................................................... 47 6.2.2. Hammock Replacement ............................................................................................................................................. 47 7. Troubleshooting ................................................................................................................................................................... 48 7.1. Quick Troubleshooting Guide ............................................................................................................................................ 48 8. Appendices ........................................................................................................................................................................... 49 8.1. Technical Specifications..................................................................................................................................................... 49 8.1.1. Environmental Requirements ..................................................................................................................................... 49 8.1.2. Mechanical Specifications .......................................................................................................................................... 49 8.1.3. Electrical Specifications .............................................................................................................................................. 49 8.1.4. Irradiance Specifications ............................................................................................................................................ 49 8.1.5. Miscellaneous ............................................................................................................................................................. 50 8.2. Compliance ........................................................................................................................................................................ 51 8.2.1. Compliance Directive: ................................................................................................................................................ 51 8.3. Trademarks ........................................................................................................................................................................ 51 8.4. Manufacturer .................................................................................................................................................................... 52 Novos Bilisphere 360 LED User Manual V1.1 1. Safety Information 1.1. Symbols BF Type Equipment Do not place your hands! Risk of Electric Shock! Use Eye Protection Mask! I ON O OFF Please see the auxiliary documents! 1 Novos Bilisphere 360 LED User Manual V1.1 1.2. Operator's Responsibility for Patient Safety WARNING! Strictly follow this manual. Any use of the product requires full understanding and strict observation of all portions of these instructions. This equipment is only to be used for the purpose specified under "Intended Use". Observe all WARNINGS and CAUTIONS as rendered throughout this manual and on labels on the equipment. The design of this equipment, the accompanying literature, and the labeling on the equipment take into consideration that the purchase and use of this equipment are restricted to trained professionals, and that certain inherent characteristics of the equipment are known to the trained operator. Instructions, warnings, and caution statements are limited, therefore, largely to the specifics of the Novos design. This publication excludes references to various hazards which are obvious to a medical professional and operator of this equipment, to the consequences of product misuse, and to potentially adverse effects in patients with abnormal conditions. Product modification or misuse can be dangerous. NOVOS Medical Systems disclaims all liability for the consequences of product alterations or modifications, as well as for the consequences which might result from the combination of this product with other products whether supplied by Novos or by other manufacturers if such a combination is not endorsed by NOVOS Medical Systems 1.3. Patient Monitoring The operators of this phototherapy system must recognize their responsibility for choosing appropriate safety monitoring that supplies adequate information on equipment performance and patient condition. Patient safety may be achieved through a wide variety of different means ranging from electronic surveillance of equipment performance and patient condition to simple, direct observation of clinical signs. Responsibility for the selection of the best level of patient monitoring lies solely with the equipment operator. 1.4. Limitation of Liability NOVOS Medical Systems’ liability, whether arising out of or related to manufacture and sale of the goods, their installation, demonstration, sales representation, use, performance, or otherwise, including any liability based upon NOVOS Medical Systems’ Product Warranty, is subject to and limited to the exclusive terms and conditions as set forth, whether based upon breach of warranty or any other cause of action whatsoever, regardless of any fault attributable to NOVOS Medical Systems and regardless of the form of action (including, without limitation, breach of warranty, negligence, strict liability, or otherwise). NOVOS Medical Systems shall not be liable for, nor shall buyer be entitled to recover any special incidental, or consequential damages or for any liability incurred by buyer to any third party in any way arising out of or relating to the goods. 2 Novos Bilisphere 360 LED User Manual V1.1 1.5. Warranty All Novos products are guaranteed to be free of defects for a period of one year from date of delivery. The following are exceptions to this warranty: 1. 2. 3. 4. 5. 6. 7. 8. The defect shall be a result of workmanship or material. Defects caused by misuse, mishandling, tampering, or by modifications not authorized by NOVOS Medical Systems or its representatives are not covered. Rubber and plastic components and materials are warranted to be free of defects at time of delivery. Any product which proves to be defective in workmanship or material will be replaced, credited, or repaired with NOVOS Medical Systems holding the option. NOVOS Medical Systems is not responsible for deterioration, wear, or abuse. In any case, NOVOS Medical Systems will not be liable beyond the original selling price. Failure caused by force-majeure events and others where the manufacturer cannot be liable. Failures due to the voltage difference Lack of or insufficient service and maintenance by the customer. Normal wear and tear of operating parts. Misuse of the machine and its equipment The buyer assures that all service & maintenance intervals are observed and protocoled by qualified personnel according to the NOVOS service manuals. All warranty liabilities expire should these obligations not be met. Application of this warranty is subject to the following conditions: 1. 2. 3. 4. NOVOS Medical Systems or its authorized representative must be promptly notified, in writing, upon detection of the defective material or equipment. Defective material or equipment must be returned, shipping prepaid, to Novos or its factory authorized service center. Examination by Novos or its factory authorized service center must confirm that the defect is covered by the terms of this warranty. Notification in writing, of defective material or equipment must be received by Novos or its factory authorized service center no later than two (2) weeks following expiration of this warranty. The above is the sole warranty provided by NOVOS Medical Systems No other warranty expressed or implied is intended. Representatives of Novos are not authorized to modify the terms of this warranty. NOVOS Medical Systems 3 Novos Bilisphere 360 LED User Manual V1.1 2. Intended Use 2.1. Applications BILISPHERE 360™ is routinely used in treatment of neonatal hyperbilirubinemia, where the patient is submitted to an exposure of concentrated radiation in the blue spectrum of visible light during a period to be determined by the attending physician depending on the case. During the jaundice treatment, the patient does need thermotherapy equipment and accommodated in a heated crib or incubator, depending on her clinical conditions. Considering the situations, NOVOS™ developed BILISPHERE 360 ™ phototherapy. The aim is supplying maximum patient comfort during the treatment and reducing the treatment duration. BILISPHERE 360 ™ is a multidirectional phototherapy especially recommended for a rapid reduction in serum bilirubin levels thus reducing the need for blood transfusion and reducing treatment duration. Lifetime of Bilisphere 360™ is 7 years. 2.2. Restrictions of Use WARNING! DANGER! Risk of explosion if used in the presence of flammable anesthetics. This device is neither approved nor certified for use in areas where combustible or explosive gas mixtures are likely. WARNING! This device is designed to be used only in rooms with line power installations complying with national safety standards for hospital patient rooms (e.g., IEC/EN 601.1, "Safety of Medical Equipment"). To maintain grounding integrity, connect only to a "hospital grade" receptacle. WARNING! The use of this device requires continuous supervision of the infant by trained nursing personnel in order to avoid immediate corrective action in situations with a risk of patient injury. WARNING! Mobile telephones must not be used within 10 meters (33 feet) of the incubator. Mobile telephones can interfere with the function of electro medical equipment and therefore endanger the patient! WARNING! Novos medical equipment conforms to the interference immunity requirements laid down in product-specific standards or in EN 60601-1-2 (IEC 60601-1-2). However, depending on the design of a mobile phone and the use situation, field strengths exceeding the values laid down in the specified standards may be generated in the immediate vicinity of mobile phones, thereby causing interference and malfunctions. WARNING! Intensive phototherapy BILISPHERE 360 does not replace an incubator or/and any other heated system. WARNING! BILISPHERE 360 is ready for operation only when all checks have been carried out successfully. WARNING! NOVOS cannot warrant or endorse the safe performance of third party accessories for use with BILISPHERE 360 ™ phototherapy. Only use original NOVOS accessories and parts with BILISPHERE 360™ phototherapy. Serious harm to the patient and device may result from the use of unauthorized parts or accessories. WARNING! BILISPHERE 360 is ready for operation only when all checks have been carried out successfully. 4 Novos Bilisphere 360 LED User Manual V1.1 3. Parts and Controls 3.1. Isometric View 5 6 1 2 7 3 8 4 1 2 3 4 Control Panel Infant Bed Handle Height Adjustment Membrane Switch Castors with Brakes 5 6 7 8 I-V Pole (Optional) Monitor Tray Side Handle Power Input, Fuse Holder, On/Off Switch 5 Novos Bilisphere 360 LED User Manual V1.1 3.2. Front View 6 Novos Bilisphere 360 LED User Manual V1.1 3.3. Side View 7 Novos Bilisphere 360 LED User Manual V1.1 3.4. Control Panel 3.4.1.Main Screen Bilisphere 360 has a 5.1” Touchscreen display on which; • • • • • • • Air Temperature Skin Temperature Therapy Time Set Therapy Time FAN Statuses Upper and Lower Skin Temperature Limits Alarms can be monitored simultaneously. 8 Novos Bilisphere 360 LED User Manual V1.1 3.5. Temperature Sensors Air and skin temperatures are measured by the corresponding sensors shown in figure below. 1 2 1 ) Skin Temperature Probe 2 ) Air Temperature Probe 9 Novos Bilisphere 360 LED User Manual V1.1 4. Preparation 4.1. Unpacking and Assembly Bilisphere 360 is packed as two main parts, which are the main body, and the accessories box containing the hammock, monitor tray, power cable, IV pole (optional) and eye protection masks. Upon receiving the device follow the steps below to complete the installation. • Unpack the device and move the main body aside. • Unpack the accessories box. Extract; o the monitor tray o power cable o the IV pole (optional) o eye protection masks • Align the monitor tray on its position. 10 Novos Bilisphere 360 LED User Manual • Insert 4 M4x12 YHB screws. • Insert white screw covers. • Slide open the infant bed drawer. V1.1 11 Novos Bilisphere 360 LED User Manual • Place the hammock. • Place the IV pole to its place and tighten the plastic head screw to fix it at desired position. V1.1 12 Novos Bilisphere 360 LED User Manual V1.1 4.2. Electronical Operation Checkout Before commencing ensure: • • • A baby is not present on the warmer. Air and Skin temperature sensors are NOT CONNECTED into their socket. The power cord is plugged into an appropriate wall supply outlet. The following procedure can be used to verify the electronical operation of Bilisphere 360. 1. 2. Press the On/Off Switch located at the right side of the device. Wait for the initialization. 3. Observe that there are two alarms for the absent air and skin probes on the main screen. 4. Insert the Air Probe into its socket. 13 Novos Bilisphere 360 LED User Manual 5. Observe that the warning message for the absent air probe is removed and the air temperature measured by air probe can be seen at the upper left region of the screen. 6. 7. Insert Skin Probe into its socket. Observe that the warning message for the absent skin probe is removed and the temperature measured by skin probe can be seen at the upper right region of the screen. 8. 9. Slide out the infant bed drawer. Observe the warning message for the opened bed. V1.1 10. Push the infant bed drawer into the device completely. 11. Observe that the warning message for opened baby bed is removed. 14 Novos Bilisphere 360 LED User Manual V1.1 12. Press on MENU button. 13. Press on LAMP button to switch to lower lamp groups. 15 Novos Bilisphere 360 LED User Manual V1.1 14. Press on NEXT button to pass to the target therapy time settings. 15. Press on NEXT button to exit from the menu. 16. Press on START button to start the therapy with only the lower lamp groups as set from menu. 16 Novos Bilisphere 360 LED User Manual V1.1 17. Check that all 8 lamps of the lower lamps group are working properly. 18. Press on STOP button and observe the therapy is terminated. 19. Press on MENU button 20. Press on LAMP button to switch to upper lamp groups. 17 Novos Bilisphere 360 LED User Manual V1.1 21. Press on NEXT button to pass to the target therapy time settings. 22. Press on NEXT button to exit from the menu. 23. Press on START button to start the therapy with only the upper lamp groups as set from menu. 18 Novos Bilisphere 360 LED User Manual V1.1 24. Check that all 8 lamps of the upper lamps group are working properly. 25. Press on STOP button and observe the therapy is terminated. 26. Press on the Up Arrow and observe increase in the height of the upper couple. 19 Novos Bilisphere 360 LED User Manual V1.1 27. Press on the Down Arrow and observe the decrease in the height of the upper couple. 20 Novos Bilisphere 360 LED User Manual V1.1 5. Operation 5.1. Switching Bilisphere 360 On • Plug the power cable to Power Inlet Socket located at the right side of the device. (1) 2 • Turn on the device by switching the On/Off button to I position (2). 1 • Wait for the device Initialization. 21 Novos Bilisphere 360 LED User Manual V1.1 1. Wait for the device Initialization. 2. Main Screen appears as shown in figure. 22 Novos Bilisphere 360 LED User Manual V1.1 5.2. Control Panel Operation Bilisphere 360 has a control panel hosting the mainboard and a 5.1” Touchscreen LCD. All the user inputs except for the manual height adjustment is transferred and processed via control panel. 5.2.1.Main Screen 1 2 3 4 5 6 7 12 11 10 1 2 3 4 5 Air Temperature Fan Statuses Temperature Unit Therapy Counter Skin Temperature 6 Upper and Lower Alarm Limits of Skin Temperature 7 Target Therapy Duration 8 9 MENU Button START Button 10 Alarms and Warning Messages 11 AUDIBLE Button 12 Audible Alarm Indicator 9 8 Air temperature measured inside the couple. Working status of the upper and lower fans. Displayed temperature unit. Time elapsed in phototherapy. Skin temperature measured by the skin probe. Desired range for the skin temperature. An audible alarm is triggered if the measured value is not in this range. The duration after which phototherapy is terminated automatically. Button to access the menu. Button to start the phototherapy. Region on which various alarm and warning messages shown. Button to turn on/off the audible alarms. Indicator for enabled/disabled audible alarm. 23 Novos Bilisphere 360 LED User Manual V1.1 5.2.2.Alarms and Warnings High Skin Temperature: The measured skin temperature is not in between specified limits. Alarm Message Warning Message Low Skin Temperature: The measured skin temperature is not in between specified limits. Alarm Message Warning Message 24 Novos Bilisphere 360 LED User Manual V1.1 Low Air Temperature: The measured air temperature is under 30⁰C. Alarm Message Warning Message 37⁰C Air Temperature: The measured air temperature is above 37⁰C. Alarm Message Warning Message 25 Novos Bilisphere 360 LED User Manual V1.1 5.2.2.1. Warning Messages Push the baby bed all the way in: The baby bed drawer is opened. Close the baby bed drawer completely. Replace lamps: Service life of the lamps is over. (Operation Time > 20.000 hours) Upper couple is rising: Height of the upper couple is being increased by thermoelevation. 26 Novos Bilisphere 360 LED User Manual V1.1 5.2.2.2. Activation/Deactivation of Audible Alarms • Press on AUDIBLE button to silence audible alarms. • Audible alarm is silenced for 5 minutes. 27 Novos Bilisphere 360 LED User Manual V1.1 5.2.3.Settings Menu Display language selection, lamp lifetime tracking, temperature unit selection and the touchscreen calibration can be made from settings menu. Follow the below steps in order to access the settings menu; • Press on SETTINGS button while initialization is in progress. • Settings screen is shown with 5 main tabs. 5.2.3.1. Changing the Display Language In settings menu the first available tab is Language tab. • Press UP or DOWN buttons to select the desired language between the options; Turkish, English, French and Spanish. 28 Novos Bilisphere 360 LED User Manual V1.1 5.2.3.2. Total Lamp Usage Time • Press on Lamp Tab to see the total usage time of the lamps. 5.2.3.3. Changing the Temperature Unit • Press on (⁰C) or (⁰F) tab button. • Press on UP or DOWN buttons to toggle between Celsius and Fahrenheit temperature units. 29 Novos Bilisphere 360 LED User Manual V1.1 5.2.3.4. Touchscreen Calibration • Press on T.Calib tab. • Press and hold the point specified with a plus sign. 30 Novos Bilisphere 360 LED User Manual • • V1.1 Release your finger when the progress bar shows 100%. Repeat the same operation for totally three different points. • After identification of third point “Calibration completed” message shown meaning that the calibration is done successfully. • Press on CONFIRM button to apply the changes. 31 Novos Bilisphere 360 LED User Manual V1.1 5.2.3.5. DEMO Mode • Press on DEMO tab. • After pressing DEMO button on the settings menu, device is re-initialized in DEMO mode. DEMO sign is seen on the upper left corner of the screen. • In DEMO Mode; • • • • • • • • Device simulates the air temperature for a better understanding of Thermo-Elevation feature. Air temperature is automatically increased up to 37°C. When air temperature is reached to 32°C, upper fan group is activated. When air temperature is reached to 34°C, lower fan group is activated. When air temperature is reached to 35°C, upper couple is risen 10cm above. When air temperature is reached to 36°C, upper couple is risen 10cm more. When air temperature is reached to 37°C, therapy is terminated. After termination of therapy, air temperature is again simulated as 30°C. A new demo session can be started by pressing the START button. 32 Novos Bilisphere 360 LED User Manual V1.1 5.3. How to Start a Therapy Before commencing the therapy, Bilisphere 360 phototherapy unit should be positioned properly. Press on the outer edge of the brake pedal to lock the brakes. • Lock the brakes of the casters to avoid undesired movements of the equipment. • Open the infant bed drawer and place the infant. • Attach the skin probe as described in Attachment/Detachment of Skin Probe section. • Attach a suitable sized eyeprotector to the infant eyes. 33 Novos Bilisphere 360 LED User Manual • V1.1 Push the bed drawer completely into the cabin. Note: Infant bed drawer must be in closed position during the therapy. Turn on the device as described in Switching the Bilisphere 360 On section. There are three parameters to be set before commencing the phototherapy. 1 3 2 1. 2. 3. The upper and lower alarm limits for the skin temperature. Target therapy duration. Active lamp groups. All these parameters should be set with respect to the clinical conditions of the baby. Below procedure explains how to start a phototherapy. 34 Novos Bilisphere 360 LED User Manual • V1.1 Press on MENU button • Press on Up or Down arrows to switch between predefined alarm limits (31-33, 32-34, 33-35, 34-36, 35-37, 36-38, 3739). • Press on NEXT button and pass to Set Therapy Time parameter. • Press on Up or Down arrows to set the desired target therapy duration. 35 Novos Bilisphere 360 LED User Manual V1.1 • Press on LAMP button to toggle between o Use only lower lamp group. o Use only upper lamp group. o Use both upper and lower lamp groups. • Press on NEXT button to exit the menu. • Press on START button to start the therapy • Observe the “Therapy is started!” warning message. 36 Novos Bilisphere 360 LED User Manual V1.1 • Press on PAUSE button to pause the therapy. Therapy counter will be stopped until it is resumed again. • Press on START button to resume the therapy. • Press on STOP button to stop the therapy. Note: Bilisphere 360 will automatically terminate the therapy after target therapy time is elapsed. 37 Novos Bilisphere 360 LED User Manual V1.1 5.4. Temperature Sensors There are one air and one skin temperature sensors located at the ceiling of the cabin of Bilisphere 1 2 360. 1 ) Skin Temperature Probe 2 ) Air Temperature Probe WARNING! Avoid applying excessive force to the sensor sockets during mounting/dismounting processes. If a skin probe is not connected to the device; • • A dashed line will be seen on the corresponding region of the main screen. “Check skin probe!” alarm is triggered. 38 Novos Bilisphere 360 LED User Manual V1.1 If an air probe is not connected to the device; • • • A dashed line will be seen on the corresponding region of the main screen. “Check air probe!” alarm is triggered. Therapy cannot be started. 5.4.1.Attachment/Detachment of Skin Probe Attachment of Skin Probe • • • • • • • Gently clean and dry the baby’s skin where you intend to place the skin sensor. Remove the backing paper from the sensor cover. Place the white plastic side of the skin sensor in the center of the adhesive side of the sensor cover as shown. Place the skin sensor and sensor cover by keeping the metal side in contact with the baby’s skin. Gently press and hold the edges of the sensor cover to allow the hydrogel adhesive to adhere to the baby’s skin Gently lift up the edge of the sensor cover. If required moisten the edges of the sensor cover using sterile water and dampened cotton swab. Gently peel the sensor cover and skin sensor from the skin surface. Take care not to pull directly on the skin sensor wire. Detachment of Skin Probe 39 Novos Bilisphere 360 LED User Manual V1.1 5.5. Infant Bed Infant bed of Bilisphere 360 is composed of side protectors (1), a hammock (2) and the baby cot (3). For the disinfection purpose, all these components are extractable from the equipment. 1 2 3 5.5.1.Baby Cot Baby cot of Bilisphere 360 has a special drain hole through which the liquid waste can be transferred to an infant drainage bag with the help of its inclined design. Peel the cover and attach the drainage bag under drain hole of baby cot. 40 Novos Bilisphere 360 LED User Manual V1.1 5.5.2.Hammock Hammock of Bilisphere 360 provides a comfortable surface while enabling the necessary blue light interference. For disinfection purpose hammock can be washed by mild detergent and water and replaced again. 5.5.2.1. Hammock Replacement • • • Extract the hammock from the bed drawer. Unfasten the Velcro edge of the hammock Pull the metal hammock frame and slide it out. • Use the reverse procedure to re-attach the hammock to its frame. • Visually check for damage before each use and dispose the hammock if necessary. 41 Novos Bilisphere 360 LED User Manual V1.1 5.6. Thermo-Elevation With the help of Thermo-Elevation feature, undesired temperature increase inside the cabin is prevented. So, risks of hyperthermia, water loss, tissue and cell damage are prevented. 5.6.1.Operating Principle Microcontroller of Bilisphere 360 continuously measures the air temperature inside cabin and increase the height of the upper couple to provide the necessary fresh air circulation. By doing so, the temperature inside cabin is kept in a safe range and prevented from undesired increments 1 Assume that a therapy is started with an air temperature of 30.0⁰C. Upper FAN starts working. 2 Air Temperature = 32.0⁰C 42 Novos Bilisphere 360 LED User Manual V1.1 Lower FAN starts working 3 Air Temperature = 34.0⁰C Upper couple is risen 10cm above. 4 Air Temperature = 35.0⁰C 43 Novos Bilisphere 360 LED User Manual V1.1 Upper couple is risen 10cm more. 5 Air Temperature = 36.0⁰C Therapy is terminated. 6 Air Temperature = 37.0C⁰ 44 Novos Bilisphere 360 LED User Manual V1.1 5.7. Manual Upper Couple Height Adjustment Upper couple height can also be adjusted manually if and only if the measured air temperature is less WARNING! If the upper couple height is increased by thermo-elevation, then user cannot decrease it unless the air temperature is not dropped under 35⁰C. than 35⁰C. Regulation of air temperature inside cabin can be assisted by manual height adjustment. Temperature values between 30-35⁰C can be obtained by changing the upper couple height. The more space between the couples, the more fresh air flows through cabin and this process avoids the undesired temperature increase. Press and hold the Up Arrow to increase the height of the upper couple. 45 Novos Bilisphere 360 LED User Manual V1.1 6. Routine Cleaning and Maintenance 6.1. General Cleaning Bilisphere 360 should be cleaned before each patient use. WARNING! To avoid the possibility of electric shock hazard when performing cleaning procedures, ensure that Bilisphere 360 is disconnected from the power supply. WARNING! Before cleaning and disinfection, elevate upper couple 20-25 cm up. WARNING! If UPS is installed ensure it is turned off prior to cleaning. Do not remove the UPS shroud during cleaning procedures. Ensure no part of the UPS is immersed in any cleaning agent. WARNING! Do not spray antiseptic through lamps and do not allow liquids to seep into equipment. WARNING! Do not use abrasive cleaning solutions, sodium hypochlorite, or alcohol for cleaning surfaces of Bilisphere 360. WARNING! Do not immerse any parts of equipment and lamps in liquid cleaners. For cleaning and disinfection of equipment the process below should be followed: • • • • • • • Elevate upper couple 20-25 cm up Disconnect power supply Using a soft brush or/and dry cloth/flannel remove the accumulated dirt from the surfaces Using a mild detergent solution and warm water clean all surface Using a dry flannel dry all surface Using an antiseptic spray disinfect all surface. Antiseptic spray must not contain alcohol. Wait for an adequate time for drying. ( Follow the instruction on the antiseptic spray label to assure perfect asepsis ) For cleaning of hammock process specified below should be followed: • • • • • Remove the hammock from its frame. Bath the hammock in a solution with a mild detergent and warm water. Rinse thoroughly in warm water. Examine the hammock for excessive worn-out or damage. Discard and replace with a new hammock if necessary. Allow the hammock to dry before reinstallation. 46 Novos Bilisphere 360 LED User Manual V1.1 6.2. Maintenance This equipment must be inspected and serviced at regular 1 year intervals. A record must be kept on this preventive maintenance. We recommend obtaining a service contract with NOVOS Service through your vendor. Expected life of LED lamps is about 20000 hours, however it is recommended to follow up the irradiance constantly with a radiation monitor to assure a better evaluation of the actual efficacy of the lamps. The irradiance source should be replaced when reaches a loss of 25% of the total WARNING! Only qualified personnel should carry out service and maintenance procedures. WARNING! To avoid the possibility of electric shock hazard when performing maintenance procedures, ensure that Bilisphere 360 is disconnected from the power supply. WARNING! Only use original NOVOS lamps to assure better performance and safety of the equipment. irradiance for bilirubin (Ebi). 6.2.1.Lamp Replacement Novosoft gives an informational message when the total time counter reaches 19.800 hours. Contact NOVOS technical service for replacement of the lamps 6.2.2.Hammock Replacement Hammock might be worn out in time, therefore regularly examine the hammock for excessive wornout or damage. Replace it if necessary. For the replacement procedure please refer to 5.5.2.1 Hammock Replacement section. 47 Novos Bilisphere 360 LED User Manual V1.1 7. Troubleshooting 7.1. Quick Troubleshooting Guide Fault The device does not turn on. Cause Power supply failure. Connect the power cord to an appropriate power outlet.. Main on/off switch in the off position. Position switch to I (on). Burnt fuse. Internal electrical problem. Screen is not turned on. Touchscreen does not function properly. Therapy does not start. Contact a certified NOVOS technical service. Internal electrical problem. Contact a certified NOVOS technical service. Touchscreen calibration is missing. Restart device and redo touchscreen calibration from settings menu. Air probe is not connected. Connect air probe. Baby bed drawer may not be placed all the way in properly. Close baby bed drawer completely. Faulty software. Lamps do not light up. Proposal Air temperature is over 37⁰C. Internal electrical problem. Contact a certified NOVOS technical service. Wait compartment air temp to decrease below 37⁰C. Contact a certified NOVOS technical service. Defective motor. Upper couple does not elevate. Fan does not work. Faulty software. Blown fuse on the motor driving board. Faulty fan. Faulty software. Contact a certified NOVOS technical service. Contact a certified NOVOS technical service. 48 Novos Bilisphere 360 LED User Manual V1.1 8. Appendices 8.1. Technical Specifications 8.1.1.Environmental Requirements Operating Temperature Operating Humidity Storage Temperature Storage Humidity 23°C - 28°C 5-99% RH, non-condensed. -20°C - 50°C 0-99% RH, non-condensed. 8.1.2.Mechanical Specifications Height Width Depth Weight Display Type Display Size Hammock Size Maximum Load Capacity 140 - 159cm 63cm 78cm 68kg Monocolor LCD 5.1" 58cm x 38cm Bassinet: 10 kg Monitor Tray: 5 kg 8.1.3.Electrical Specifications Supply Voltage and Current Applied Parts Fuses Sopply Frequency Maximum Power Consumption Noise Level 220 VAC( ± % 10 ), 1.21A Class 2 Type BF 2A 50-60 Hz 270VA <55dBA 8.1.4.Irradiance Specifications Number of LED Tubes Spcetral Irradiance Dominant Wavelength Interval LED Tubes Lifetime 16 >100µW/cm^2/nm 420-480nm 20.000 hours 49 Novos Bilisphere 360 LED User Manual V1.1 8.1.5.Miscellaneous LED Lifetime Counter Reset Option Casters Fan Target Therapy Duration Skin Temperature Alarm Upper Limits Skin Temperature Alarm Lower Limits Skin Probe Failure Alarm Therapy Ended Alarm LED Lifetime Warning High Air Temperature Warning Low Air Temperature Warning Air Temperature Display Range Skin Temperature Display Range Interface Language Yes 2 with brakes, totally 4 casters 2 units 0-99 hours. Adjustable with increments of 1 hour. 33 - 39°C. Adjustable with increments of 1°C. 31 - 37°C. Adjustable with increments of 1°C. Yes Yes Yes >37°C <28°C 0°C - 51°C 0°C - 51°C Turkish, English, French, Spanish 50 Novos Bilisphere 360 LED User Manual V1.1 8.2. Compliance The NOVOS Bilisphere 360™ phototherapy units are designed to conform to requirements of: IEC 60601-1 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance IEC60601-2-50 Medical electrical equipment - Part 2-50: Particular requirements for the basic safety and essential performance of infant phototherapy equipment ISO 13485:2003 Specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services. The primary objective of ISO 13485:2003 is to facilitate harmonized medical device regulatory requirements for quality management systems. As a result, it includes some particular requirements for medical devices and excludes some of the requirements of ISO 9001 that are not appropriate as regulatory requirements. Because of these exclusions, organizations whose quality management systems conform to this International Standard cannot claim conformity to ISO 9001 unless their quality management systems conform to all the requirements of ISO 9001. 8.2.1.Compliance Directive: Council Directive 93/42/EEC of 14 June 1993 concerning medical devices (OJ No L 169/1 of 1993-0712) The Medical Device Directive (Council Directive 93/42/EEC of 14 June 1993[1] concerning medical devices, OJ No L 169/1 of 1993-07-12) is intended to harmonize the laws relating to medical devices within the European Union. The MD Directive is a 'New Approach' Directive and consequently in order for a manufacturer to legally place a medical device on the European market the requirements of the MD Directive have to be met. Manufacturers' products meeting 'harmonized standards'[2] have a presumption of conformity to the Directive. Products conforming with the MD Directive must have a CE mark applied. The Directive was most recently reviewed and amended by the 2007/47/EC and a number of changes were made. Compliance with the revised directive became mandatory on March 21, 2010. 1984 8.3. Trademarks Bilisphere 360™ and Novosoft™ are trademarks of Novos Tıbbi Cihazlar Sanayi ve Ticaret İthalat ve İhracat Limited Şirketi. 51 Novos Bilisphere 360 LED User Manual V1.1 8.4. Manufacturer Novos Tıbbi Cihazlar Sanayi ve Ticaret İthalat ve İhracat Limited Şirketi Address: Uzay Cagi Cad. Ayik Is Merkezi No: 82-B5 Ostim- ANKARA TÜRKİYE Tel: Fax: +90.312.384 15 88 +90.312.384 15 98 E-mail: info@novos.com.tr 52