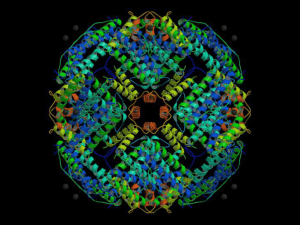
BITS Pilani Pilani Campus CHEM F343: Inorganic Chemistry III Lecture-6 1/29/2024 BITSPilani, Pilani Campus Transport of metal ions in vivo • At physiological O2 concentration, Fe2+ is readily oxidized to Fe3+, which is highly insoluble in aqueous solution at neutral pH Fe3+ (aq) + 3OH- (aq) Fe(OH)3 (s) Ksp ~ 10-39 M4 • To overcome such serious solubility problem, trace transition metals require special carrier proteins to bind and transport the ions in vivo 1/29/2024 BITSPilani, Pilani Campus Iron transport: Transferrin • Serum transferrin, melanotransferrin ovotransferrin, lactoferrin and • Lactoferrin is found in milk, while • Ovotransferrin in egg white • Tf isolated from blood plasma is occasionally referred as serotransferrin while Ovotransferrin as Conalbumin • Tf are glycoproteins, that bind two Fe(III) ions in similar but structurally distinct sites termed as N-terminal and Cterminal domains 1/29/2024 BITSPilani, Pilani Campus Transferrin: a protein 1/29/2024 BITSPilani, Pilani Campus Features of Tf during iron binding • The iron binding site is rich in hard ligands, suitable for Fe(III) binding • When the iron atom enters the active site, it is coordinated by one η1-aspartyl, one histidyl and two tyrosinate side-chains; a non-protein ligand carbonate (CO32−) • Carbonate group is held in place within the protein via hydrogen bonding interactions • The carbonate coordinates to the iron in an η2 fashion; in other words, it is a chelating ligand. 1/29/2024 BITSPilani, Pilani Campus Overall structure of Tf • Both iron atoms gets deeply buried within the protein structure, which might be protecting the iron atom from microbial siderophores 1/29/2024 BITSPilani, Pilani Campus Iron transport • Carbonate ion is required for strong Fe3+ coordination • Neither Fe3+ nor CO32- binds Tf strongly on its own, but together binding favours • Such cooperative behavior is termed as synergism 1/29/2024 BITSPilani, Pilani Campus UV-Visible studies of Fe-Tf (a) Tf at pH 6 (A) (b) Fe-Tf at pH 6 (B) (c) Tf at pH 11 (C) (d) Fe-Tf at pH 11 (D) 245nm Fe3+ + Tf + CO32- (Fe3+)Tf(CO32-) + 3H+ ~103 s-1 3H+ are from Tyr and Asp. Step wise: Fe2+ + Tf + CO32(Fe2+)Tf(CO32-) + xH+ ~107 s-1 (Fe2+)Tf(CO32-) +O2 (Fe3+)Tf(CO32-) + O2- •Tf exhibits two absorption bands •Fe binding enhances the absorption Absorption spectrum of native transferrin showing the tyrosinate-Fe3+ charge transfer band that develops at 245nm 1/29/2024 BITSPilani, Pilani Campus UV-Visible studies of Fe-Tf UV-VIS spectroscopy can be convenient method The red color of the Fe3+ protein: due to a tyrosinate → Fe3+ LMCT There is also an increased absorbance in the UV region ca 245 nm and 295nm from the deprontonated tyrosine • Change in absorbance monitored at 245 nm Titration of Conalbumin (Tf) with Fe(III) 1/29/2024 • Reveals the binding of two iron/Tf BITSPilani, Pilani Campus Iron transport • Since all the coordinating atoms in transferrin is hard in nature, Tf forms stable complex with iron(III). • The stability constant of the Fe(III)–transferrin complex is in the order of 1020 mol−1 • Strong stability constant protects the iron(III) against the low concentration of any siderophores present • Transferrins show mild antibacterial properties to prevent extensive iron chelation by siderophores • Transferrin also forms relatively stable complexes with other hard metals 1/29/2024 BITSPilani, Pilani Campus Stability constants of M-Tf complexes Metal Log10(stability constant) Cadmium(II) 5.95 Zinc(II) 7.80 Aluminium(III) 13.50 Iron(III) 22.80 • These data suggests that other metals can also be transported by transferrin into cells, where they are potentially harmful. • Aluminium, is of major concern because it is used widely in cooking utensils • Accumulation of Aluminium leads to Alzheimer's disease 1/29/2024 BITSPilani, Pilani Campus Iron translocation by Tf • Endocytosis: Process by which cell breaks off to form an internal vesicle • Membrane bound H+-ATPase pumps the protons into the vesicle to drop the pH by 5.5 to release iron •The free iron then being transported to the cytosol aqu. Component of cytoplasm through the channels •Fe can then be used for heme formation, stored in ferritin or bound by other enzymes •Fusion with cell membrane and release of apo-Tf 1/29/2024 BITSPilani, Pilani Campus Metal Storage: Ferritins Class of storage protein 1/29/2024 BITSPilani, Pilani Campus Metal Storage: Ferritins • In mammals, iron is stored in liver, spleen and bone morrow • An average person contains 3-4 gm of iron, 35 mg is used biological process like oxygen transport, redox and enzymatic reactions • Major part of Fe is stored in liver, spleen and bone marrow for mammals. • Ferritins are a class of iron storage proteins found among the animal, plant and microbial kingdoms • Ferritin is the major repository for iron storage in eukaryotes • This avoids the buildup of large quantities of free iron with accompanying solubility problems and provides a reserve of iron in a stable form that is readily accessible 1/29/2024 BITSPilani, Pilani Campus Structure of Ferritin • Horse spleen ferritin is composed of 24 subunits assembled into hollow spherical structure within which iron is stored as a hydrous ferric oxide mineral core, can hold upto 4500 Fe3+ centers. • The core diameter lies between 60-80 Å • The structure of horse spleen ferritin and apoprotein have been determined by X-ray crystallography • The figure shows a schematic drawing of the ferritin molecule and a diagram of an individual subunit showing the bundle of helices from which it derives its tertiary structure • These subunits form a shell around the hollow core that accommodates the iron ions Ferritin 1/29/2024 BITSPilani, Pilani Campus Structure of Ferritin (contd) • Access to this core is by way of a set of 8 funnel-shaped channels that possess a 3-fold axis of symmetry and are lined with hydrophilic residues (Asp, Glu, Ser) • Another series of channels possesses a fourfold axis of symmetry and are lined with 12 hydrophobic leucine residues which provide access for organic reductants (NADH- Nicotinamide adenine dinucleotide or FADH: coenzyme) or chelating agents that are required for release of iron • In common with other iron-binding ligands, ferritin takes up iron as M2+ because 1. First, Fe3+ is very insoluble at intracellular pHs 2. Second, Fe2+ is kinetically labile relative to Fe3+ [k (Fe2+) ~107s-1; k (Fe3+) ~103s-1] which is essential for rapid complexation. Structure of Ferritin (contd) • • • • • • • Iron enters the ferritin core as Fe2+, is subsequently oxidized to Fe3+ and taken up by the growing crystalline lattice within the protein core Oxidation to inert Fe3+also stabilizes iron for long–term storage or transport The ferritin core is composed of a microcrystalline array of ferrihydride phosphate [Fe(O)OH)8(FeOPO3H2)∙xH2PO4] formed by oxidation of Fe2+ and hydrolysis of Fe2+ and uptake of orthophosphate Experiments with 18O2 and H218O have shown that the oxygen atoms derive from water, and so oxidation and hydrolysis are discrete reactions 4Fe2+ + O2 + 4H+ = 4Fe3+ + 2H2O 4Fe3+ + 8 H2O = 4Fe(O)OH + 12H+ Ferritin • Ferritin possesses eight funnel-shaped hydrophilic channels • Channels are lined with hydrophilic residues like Asp, Glu and Ser • Ferritin core is composed [Fe(O)(OH)8(FeOPO3H2).xH2PO4] 1/29/2024 of ferrihydrite phosphate BITSPilani, Pilani Campus Next class ✓ Oxygen carrier and transport 1/29/2024 BITSPilani, Pilani Campus