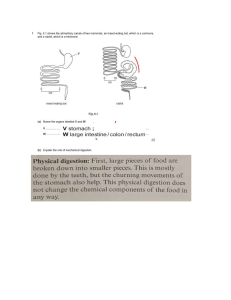
School of Health Sciences Yashwantrao Chavan Maharashtra Open University B.Sc. (MLT) S.Y.B.Sc. (MLT) HSC 124 Clinical Pathology and Biochemistry Clinical Pathology and Biochemistry Unit No. Unit Page No. Unit 1 : Laboratory Examination of Miscellaneous Body Fluids Unit 2 : Normal and Abnormal Biochemical Processes of The Body 23 Unit 3 : Routine Biochemical Tests 50 Unit 4 : Biochemical Test Profile 74 Unit 5 : Principals of Analytic Techniques 3 123 FORWORDS The program of paramedical education at diploma and degree level has been accepted by students at various study centres recognized by YCMOU in Maharashtra. The DMLT and B.Sc. are two such popular programs run by YCMOU. The paramedical technicians constitute the backbone of health care delivery system in rural and urban area. In view of the importance of the program the YCMOU is making an all out effort to provide academic support to the institutes and students by providing study material. One of the major contributions of the YCMOU is in the field of curriculum development and in the development of model instructional materials. The material is developed in consultation with subject experts, teachers and employers. The present volume on Clinical Pathology and Biochemistry is meant for the students of second year B.Sc. (MLT). It is being published for wider distribution amongst students and teachers throughout the state. I hope the book will be useful to everyone. I am grateful to those who have contributed to the development of this volume. I must acknowledge also the interest taken by Dr. Prakash Atkare, Dr. Jaydeep Nikam, Dr. Prakash Palwe, Dr. Rajashree Mutha Dr. Abhay Patil, Dr. Uday Mahajan and Prashant Dongre. Suggestions for improvement of this volume will be welcome. Vice-Chancellor Yashwantrao Chavan Maharashtra Open University Vice-Chancellor : Prof. (Dr.) M. M. Salunkhe SCHOOL OF HEALTH SCIENCES : SCHOOL COUNCIL Dr. Jaydeep Nikam, (Chairman) Director School of Health Sciences Y. C. M. Open University Nashik Dr. R. V. Vadnere Director School of Continuing Education Y. C. M. Open University Nashik Dr. Sachin Siddeshwar Mumbare Professor Department of Community Medicine Dr. Vasantrao Pawar Medical College Hospital & Research Centre, Nashik Dr. Madhuri Sonawane Assistant Professor School of Agriculture Sciences Y. C. M. Open University, Nashik Dr. Prakash Palwe Senior Consultant School of Health Sciences Y.C.M. Open University, Nashik Dr. Pratibha Phatak Dr. Hedgewar Hospital Aurangabad Dr. Farooq F. Motiwala Principal Motiwala Homoeopathic Medical College Nashik Dr. P. B. Tahilramani Nett Paramedical College Vadawali Ghodbander Road Thane (W) AUTHOR Mrs. Vibhavari Parashare H.O.D., Dept. of Bio-chemistry K.T.H.M. College, Nashik EDITOR Dr. Jaydeep Nikam Director School of Health Sciences Y. C. M. Open University Nashik Dr. Prakash Palwe Senior Consultant School of Health Sciences Y.C.M. Open University, Nashik PRODUCTION Shri. Anand Yadav Manager Print Production Centre YCMOU, Nashik © 2014, Yashwantrao Chavan Maharashtra Open University, Nashik 422 222 n First Published by : YCMOU, Nashik (Sept. 2014) n Printed by : Shri. Narendra Shaligram, M/s Replica Printer, 2, Chitco Centre, Vakilwadi, Nashik 422 001 n Published by : Dr. Prakash Atkare, Registrar, Yashwantrao Chavan Maharashtra Open University, Nashik - 422 222 (S.Y.B.Sc. (MLT) Clinical Pathology and Biochemistry) DB/AM14-161 Unit 1 : Laboratory Examination of Miscellaneous Body Fluids Learning Objectives: • To know the different functions of CSF • To understand the importance of serous fluid Chapter 1 : Cerebrospinal fluid • Introduction CSF formed by selective dialysis of plasma by the choroid plexus of the ventricles of the brain. It is present in the cavity that surrounds the brain in the skull and the spinal cord in the spinal column. Volume is about 150 ml. Fig 1.1 Choroid plexus and CSF flow CSF Circulation • Functions of CSF: 1) It helps to protect the brain and spinal cord from injury by acting like a fluid buffer. 2) It also acts as a medium for the transfer of substances from the brain tissue and spinal cord. Clinical Pathology and Biochemistry : 1 Normal composition of CSF: Colour Ph Appearance Specific gravity Total solid Proteins Glucose Chloride Sodium Potassium Creatinine Cholesterol Urea Uric acid Lymphocytes Neutrophils Colourless 7.3-7.4 Clear 1.03-1.008 0.85-1.70 g/dl 15-45 mg/dl 40-80 mg/dl 700-750 mg/dl 144-154 m Eq/l 2.0-3.5mEq/l 0.5- 1.2 mg/dl 0.2-0.6 mg/dl 6-16 g/dl 0.5 – 4.5 mg/dl 0 – 8 /cu mm Absent • Collection of CSF: It is collected by the physician, surgeon, pathologist, anaesthetist and other specialists. The sterile lumber puncture needle is required. It is inserted between the 4 th and 5 th lumbar vertebra to a depth of 4 to 5 cm. withdraw the stylet and collect the fluid into clean sterile three bulbs or test tubes. Fig 1.2 – Collection of CSF First bulb is used for bacterial culture; do not store the sample in cold because commonly sought pathogen Neisseria meningitides is killed when exposed to cold. Centrifuge the second sample and use the sediment to prepare the three smears for gram staining, acid fast staining and for leukocyte count. • Indications of examination of CSF and Lumbar Puncture: 1. Diagnosis of meningitis – Viral bacterial tubercular, syphilitic 2. Diagnosis of disease of brain in co-relation with clinical findings – CNS malignancy) brain tumour) 3. Measurement of intra-cranial pressure 4. Lumbar puncture with installation of antibiotics is used for therapy of meningitis. 5. Repeated Lumber Puncture (LP) is advised in head injury & subarachnoid haemorrhage. 6. LP is also done for spinal anaesthesia Clinical Pathology and Biochemistry : 2 (A) Physical examination if CSF: 1) Colour 2) Appearance 3) Presence of Blood 4) Presence of clot or fibrin web. (B) Chemical examination of CSF: 1. Determination of glucose: • Method: Enzymatic, Glucose oxidase method • Principle: Glucose contains aldehyde as a functional group. The aldehyde group of glucose is oxidised by glucose oxidase enzyme to give gluconic acid and hydrogen peroxide which is broken by peroxidase enzyme in to water and oxygen. Liberated oxygen reacts with 4 -Aminophenazones in the presence of phenol to form pink coloured compound. The intensity of pink coloured compound is directly proportional to the glucose concentration in the CSF. Glucose reagent CSF Glucose standard D.W Test 3 ml 0.02 ml Standard 3 ml Blank 3 ml 0.02 ml 0.02 ml Mix and incubate for 15 minutes at 370C • Calculations: (O.D of test /OD of Std) × 100. 2. Determination of Protein: • Method: Precipitation • Principle: Proteins contain amino acids linked by peptide linkage and different functional groups. There are some chemicals which denature the protein for example acids, alkalis etc. The proteins denatured by sulphosalicylic acid loose its solubility and form turbidity. The turbidity gets precipitated. • Procedure: Take 4 ml of sulphosalicylic acid and add 1ml of CSF sample in one test tube. In second test tube take 4 ml of sulphosalicylic acid and add 1 ml of protein standard of 60mg/dl .measure the O.D at 640 nm. • Calculations: (O.D. of test / O.D. of Std.) × 60 3. Determination of CSF Chloride: • Method: Titration • Principle: In this method first precipitate the protein from the CSF sample and the protein free filtrates is obtained. After centrifugation the filtrate is titrated with mercuric nitrated using Di-phenyl-carbazone as indicator the appearance of blue violet colour is considered as the end point of the titration. Clinical Pathology and Biochemistry : 3 • Procedure: Use chloride standard concentration 100 m Eq/l Take 2 ml of protein free CSF add one drop of indicator, titrate against mercuric nitrate reagent till blue violet colour formed. Repeat same using standard instead of CSF. • Calculations: (ml of HgNO3required for CSF/ ml HgNO3 required for STD.) ×100 Pandy’s test is used to determine globulin content in which appearance of turbidity gives positive test. 4 Determination of globulins by Pandy’s test Clinical Significance: This test gives a rough idea of the extent of any increase in globulin in bacterial infection. • Pandy’s reagent: It is prepared by dissolving 10 gm of phenol in 150 ml of distilled water. It should be clear and colourless. • Procedure: 1. Pipette 2 ml of Pandy’s reagent in test tube. 2. Add 2 to 3 drops of clear CSF specimen. Do not mix. 3. Observe for the formation of turbidity. • Observation: 1. No formation of precipitate- globulin normal 2. Formation of precipitate ring – globulin increased • • Clinical significance The CSF chemical and physical examination is found to be very important in a diseases related to brain, spinal cord, nervous system. In the diagnosis of Meningitis, inflammation of the meninges the CSF examination is very important. Inflammation to the lining of the skull and to the covering of the brain and spinal column causes disturbance in the central nervous system. In such case the diagnosis depend on the CSF examination and its physical as well as chemical composition. This examination is also used in the encephalitis, spinal cord tumour, multiple sclerosis etc. The variations in the composition of the CSF infections show various changes in the appearance, chemical examinations and in the microscopic examination. (C) Microscopic examination of CSF It includes Leukocyte count, differential count, gram staining, Zn staining, India ink preparation, Giemsa staining. (1) CSF total cell counts: Red blood cell (RBC) count.- Normally no red blood cells are present in the CSF. The presence of red blood cells may indicate bleeding into the CSF or may indicate a "traumatic tap" - blood that leaked into the CSF sample during collection. White blood cell (WBC) count.- Normally less than 5 cells are present in the adult. A significant increase in white blood cells in the CSF is seen with infection or inflammation of the central nervous system. Clinical Pathology and Biochemistry : 4 CSF cytology – a cyto-centrifuged sample is treated with a special stain and examined under a microscope for abnormal cells. This is often done when a central nervous system tumour or metastatic cancer is suspected. The presence of certain abnormal cells, such as tumour cells or immature blood cells, can indicate what type of cancer is involved. • (2) Total Leukocyte count (WBC) of CSF: Procedure: Mix the CSF sample. If the sample is Clear CSF do not dilute the sample. In WBC pipette take CSF up to 0.5 marks and dilute with the CSF diluting fluid up to 11 marks. CSF diluting fluid contains acetic acid and methylene blue which removes the red blood cells and stains leukocytes. Mix well and wait for 10 minutes. Load the well mixed sample on Neubauer chamber. Focus under low power (10X) and adjust condenser and diaphragm for maximum visualization. Switch to high dry (45X), adjust if necessary, and count cells. For an undiluted sample, usually all 9 squares are counted. Count the cells in all 9 square i.e. area counted = 9 mm2 • Calculations: = • ( ) ( )× Leukocytes in CSF /cu mm = × If CSF sample is turbid dilution method is used. In this case dilute the CSF 1:20 and then use in the procedure as describe above. The calculation of diluted sample is as follows. × × Leukocytes in CSF /cu mm = (3) Differentials count • Procedure. It may be performed on a stained smear usually Wright’s stain using CSF. It is recommended that stained smears be made even when the total cell count is within normal limits. Count 100 cells in consecutive oilpower fields. Report the percentage of each type of cell present. Normal CSF Differential Cell Count is as follows: Cell type Adults Neonates Lymphocytes 60% ±20% 20% ± 15% Monocytes 30% ± 15% 70% ± 20% Neutrophils 2% ± 4% 4% ± 4% Clinical Pathology and Biochemistry : 5 (4) Gram staining: Gram stain is positive in 60 to 80 percent of untreated cases of bacterial meningitis and in 40 to 60 percent of partially treated cases. • Procedure: Make smear of centrifuged CSF and cover with crystal violet stain for 1 minute. Wash under tap water. Flood the smear with Gram’s iodine solution for one minute. Drain it. For 30 seconds decolorize the smear with alcohol – acetone and wash the slide under running tap water. Counter stain Safranin is used. Spread it on the smear for 10 second and drain it, let it dry. Observe under high power and finally under oil immersion objective. Look for the following, Gram negative intracellular diplococcic : N. meningitides Gran positive diplococcic : S. pneumonia Gram negative rods : H. influenzae Gram positive streptococci : S. agalactiae (5) Ziehl-Neelsen staining: Acid-fast staining should be done if tuberculosis is clinically suspected. • Procedure: Prepare the smear and heat fixes it. Flood the smear with carbol – fuchsin stain, heat gently by Bunsen burner flame until steam rises, wait for 3-5 minutes.Wash under tap water. Decolorize the slide using 20% Sulphuric acid for one minute. Counterstain the slide using methylene blue for one minute. Wash under tap water, allow it to dry and observe the slide under low power objective and examine under oil immersion objective. Look for the following, Acid fast organisms: Bright red bacilli on blue back-ground. (6) India Ink Preparation: This technique is used in the identification of Cryptococcus neoformansin CSF. If the Cryptococcal meningitis is suspected the specimen should not be centrifuged and used for India ink staining. • Procedure: India ink contains tricresol. Place the drop of India ink on a clean and dry slide, add one drop of CSF mix and place cover-slip. Examine under high and oil immersion objective .Cryptococcus neoformans appears as a clear disc against a black background. This is because Cryptococcus neoformansis encapsulated and India ink fails to stain the capsule. (7) Giemsa Stain: This staining is performed if Burkitt’s lymphoma is suspected to detect morula cells. These can be found when Trypanosomes have invaded the central nervous system. • Procedure: Prepare the smear of CSF deposit and flood the smear with Giemsa stain. Wait for two minutes and add equal amount of buffer of PH 7. Wait for 10 minutes. Wash under tap water. Let it drain and observe under oil immersion for morula cells. Clinical Pathology and Biochemistry : 6 (C) Cultural Examination of CSF: If CSF contains cells and total protein is raised cultural examinations of CSF are necessary. The different agars used for culturing of CSF are, a) Chocolate agar plate b) Mac-Conkey agar c) Blood agar plate d) Sabouraud agar e) Löwenstein–Jensen medium (L.J) Medium slope Inoculate chocolate agar and examine the plate for Neisseria meningitides, Hemophilus influenzae, and Streptococcus pneumonia. Examine the blood agar plate and Mac-Conkey agar plate forE.Coli, Stap-aureus. Examine the Sabouraud agar plate for Cryptococcus neoformans. (D) Serological Examination of CSF: In Syphilis – VDRL test is performed using CSF sample. (Because in blood it may be negative) (E) Chemical examination of CSF: (1) Determination of glucose: • • Method: Enzymatic, Glucose oxidase method. Principle: Glucose contains aldehyde as a functional group. The aldehyde group of glucose is oxidised by the enzyme glucose oxidase to give gluconic acid and hydrogen peroxide which is broken by peroxidase enzyme in to water and oxygen. Liberated oxygen reacts with 4 -Aminophenazones in the presence of phenol to form pink coloured compound. The intensity of pink coloured compound is directly proportional to the glucose concentration in the CSF. Glucose reagent CSF Glucose standard D.W Test 3 ml 0.02 ml Standard 3 ml Blank 3 ml 0.02 ml 0.02 ml Mix and incubate for 15 minutes at 370C • Calculations: (O.D. of test /O.D. of Std) × 100 Clinical Pathology and Biochemistry : 7 (2) Determination of Protein: • • Method: Precipitation. Principle: Proteins contain amino acids with different functional groups. There are some chemicals which denature the protein for example acids, alkalis etc. The proteins are denatured by sulphosalicylic acid and loose its solubility and form turbidity. The turbidity get precipitated. • Procedure: Take 4 ml of sulphosalicylic acid and add 1ml of CSF sample in one test tube. In second test tube take 4 ml of sulphosalicylic acid and add 1 ml of protein standard of 60mg/dl. Measure the Optical density (O.D) at 640 nm. • Calculations: (Optical density of test / Optical density of Standard) × 60 (3) Determination of CSF Chloride: • Method: Titration • Principle: In this method first precipitate the protein from the CSF sample and the protein free filtrates is obtained. This filtrate is titrated with mercuric nitrated using Diphenylcarbazone as indicator the appearance of blue violet colour is considered as the end point of the titration. • Procedure: Use chloride standard concentration 100 m Eq/l Take 2 ml of protein free CSF add one drop of indicator, titrate against mercuric nitrate reagent till blue violet colour formed. Repeat same using standard instead of CSF. • Calculations: (ml of HgNO3 requires for CSF/ ml HgNO3 require for standard.) ×100 Pandy’s test is use to determine globulin content in which appearance of turbidity gives positive test. • Clinical conditions and CSF( clinical significance): No. Test Normal Abnormal Clinical Condition I. Physical 1 2 3 Colour Appearance Presence of Blood Colourless Cloudy or Purulent Clear Turbid Absent Present 4 Clot Absent Present Bacterial Meningitis Bacterial Infection Bleeding Subarachnoid, haemorrhage Accidental bleeding Bacterial Meningitis Clinical Pathology and Biochemistry : 8 II. Chemical 1 Glucose 50-80 mg/dl Low increase Bacterial, Viral, Fungal, Tubercular, Brain, Tumour 2 Proteins 2040mg/dl High Bacterial, Viral, Fungal, tubercular 3 Chlorides 110-130 mg/dl Decrease Bacterial, Viral, Fungal Tubercular 4 Globulin ---- Increase Bacterial Infection 1. Lymphocyte 0-5 Increase Viral & Tubercular 2. Neutrophil Absent Increase Bacterial Infection 3. RBC Absent Increase Bleeding, III. Microscopic Subarachnoid, haemorrhage Clinical App conditions Bacterial infection Cloudy Cells /cubic mm Glucose chlorides .500 neutrophils low Viral infection Clear (10-200)Mostly lymphocytes Fungal infection Brain tumour Clear Clear ( 0-5) Lymphocytes 0-5 Marked decreased Slightly Moderate low or decreased normal Low Normal Increased Normal Acute meningitis Cloudy to clot Very high count Very low Low Protein s High High Normal increase d Very high Questions For Practice : • Write short note on CSF: Collection , Examination, Significance. • List the microscopic examination of CSF. • Write a short note on the cultural examination of CSF. • Write a short note on the normal composition of CSF. Clinical Pathology and Biochemistry : 9 Chapter 2 : Serous fluids Learning Objectives: • To understand the importance of serous fluid In The laboratory many body fluids are routinely examined. They are extra-vascular fluids because they exist outside the body vessels. All are extracellular fluids. They are also termed as serous fluids. The fluids present in the body cavities come from fluid of the vascular space. Fluids are categorised into Transudates and Exudates. Transudate Exudate Increased hydrostaticpressure, Main causes Decreased colloidosmotic pressure Inflammation Appearance Clear, serous and light yellow Cloudy or clear, haemorrhagic Specific gravity < 1.012 > 1.020 Protein content < 2.5 g/L > 2.9 g/L Absent Present Effusions into different cavities Fluids collected from the infected sites Absent Spontaneously Bacteria Examples Clot Cholesterol content < 45 mg/ dL > 45 mg /dL • Serous fluids are, 1) Pleural fluid (around the lungs) 2) Pericardial fluid (Around the heart) 3) Peritoneal fluid(Around the abdominal cavity) 4) Synovial fluid (around the joints Specimen collection of these fluids is performed by the surgeon or physician. Clinical Pathology and Biochemistry : 10 Physical, Chemical and Microscopic examination of Serous fluids contains following main parameters. I. Physical examination 1. Appearance & Colour 2. Clot Formation 3. Specific Gravity Normal Clear Absent Below 1.018 II. Chemical Examination 1. Glucose 2. Protein 40-80- mg% 20-40 gm% Abnormal Cloudy, turbid Present High Low High III. Microscopic Examination 1. Clear Fluid A. TLC: Directly load the Neubauer’s chamber by sample TLC: Total cells counted in 9 squares B. DLC: Make smear, stain by Field/Leishmann 2.1 Pleural fluids: Fig: 2.1.1 Collection of Plural fluid It is obtained by per-cutaneous punctures. The fluid is collected in three sterile test tubes or bulbs. One with tube containing anticoagulant for glucose and protein determination and one is plane for clot and bacteriological test. • Normal pleural fluid has the following characteristics: It is a Clear filtrate of plasma that originates from the parietal pleura. A pH of 7.60-7.64, Protein content of less than 3% (1-2 g/ dL) Clinical Pathology and Biochemistry : 11 Fewer than 1000 white blood cells (WBCs) per cubic millimetre are present while the Glucose content similar to that of plasma and Lactate dehydrogenase (LDH) value is less than 50% of plasma. (A)Physical examination: Colour is normally pale and straw. Appearance is normally clear. Turbidity in the plural fluid it may be due to increase in the cells and debris. Inflammation due to viral or bacterial infection gives cloudy appearance to fluid. Milky plural fluid indicates Rheumatoid arthritis and tuberculosis. Clot, Normally clot is absent in the fluid. Formation of clot indicates presence of fibrinogen due to capillary wall damage. Specific gravity is below 1.018 which may be increase because of high protein content. (B) Microscopic examination: • WBC count, RBC count, Differential count. (1) Total Leukocyte count (WBC) : • Procedure: Mix the sample. If the sample is clear do not dilute the sample. Charge the Neubauer chamber directly using the clear sample and stain. Focus under low power (10X) and adjust condenser and diaphragm for maximum visualization. Switch to high dry (45X), adjust if necessary, and count cells. For an undiluted sample, usually 4 squares are counted (1, 4,7,13). Count the cells in a 4 square i.e. area counted = 4 mm2 • Calculations:= ( )× Leukocytes in pleural fluid /cu mm = ( ) × • In case of turbid Pleural fluid: In WBC pipette take pleural fluid up to 0.5 marks and dilute using saline up to 11 marks. Diluting fluid contains acetic acid so it causes turbidity by reacting high protein contents. Mix well and wait for 10 minutes. Load the well mixed sample on Neubauer chamber. Focus under low power (10X) and adjust condenser and diaphragm for maximum visualization. Switch to high dry (45X), adjust if necessary, and count cells. Usually 4 squares are counted (1, 4, 7, and 13). Count the cells in a 4 square i.e. area counted = 4 mm2 • Calculations: Leukocytes in diluted pleural fluid /cu-mm = × × (2) Differentials WBCCount: Centrifuge the plural fluid and make two smears of the sediments. Stain using wright’s stain. Count 100 cells in consecutive oil-power fields. Report the percentage of each type of cell present. Lymphocytic effusion may be in the conditions like cirrhosis, cardiopulmonary disease, infection mononucleosis while in asthma; parasitic diseases Eosinophilic effusion is observed. (C) Chemical examination: 1) Determination of Glucose: Use glucose oxidase method as describe in the CSF glucose determination. Clinical Pathology and Biochemistry : 12 Normal range: 70-110 mg/dl. The value of glucose less than 40 mg/dl may suggest bacterial infection, malignancy, non-septic inflammation. 2) Determination of protein : • Method: Biuret method • Principle: Protein in the fluid sample reacts with biuret reagent and formed purple colour complex. The intensity of which is directly proportional to the protein concentration in the serum sample. • Procedure: Standard protein concentration is 4 g/dl. Take two test tubes labelled as standard and plural fluid test. Add 5 ml of Biuret reagent in each test tube. Further add 0.05 ml of fluid and standard in respective tubes, incubate for 20 min and take the absorbance (O.D) at 530nm. • Calculations: • (Optical density of test / Optical density of Standard) × 4g /dl • Normal range: less than 3.5 g/dl Pleural Fluid Physical Odour Hot Cobweb Sp. Gravity. Normal Abnormal Quantity 10 ml Pale & Straw Colour Turbid Cloudy/Hazy Blood/ Red Milky Present present <1.018 >1.018 Absent Absent --- Condition: Pleural effusion Bacterial Infection Viral/Bacterial infection Traumatic tap Pancreatitis, , Tuberculosis Congestive heart failure Hepatic cirrhosis Intra pleural malignancy TB Bacterial Infection TB Infection Transudate Exudates Chemical Examination Glucose 70-110 mg% <40 mg% Protein --- < 3g gm% Bacterial Infection TB Infection Malignancy > 3b gm% Tranudate Exudates Clinical Pathology and Biochemistry : 13 Microscopic Examination DCLymphocyte Occ. Lymphocyte Cardio Pulmonary abscess, TB, Viral infection Eosonophil Pulmonary infection Asthma Abnormal Parasitic Disease Malignant cells Malignancy • Clinical Significance: Increased turbidity may be due to increase in cells and debris. Cloudy app. indicate inflammation due to viral or bacterial infection while bloody appearance is due to traumatic tap, pancreatitis, pulmonary infarction pleural infection, tuberculosis, hepatic cirrhosis. In bacterial infection WBC count over 1000 per cu mm. and more than 50% of neutrophil suggests inflammation due to bacterial infection. Lymphocytes increase in tuberculosis, lymphoma, carcinoma and viral infection. 2.2 Peritoneal fluid examination: It is also called ascetic fluid. It is present in the peritoneal cavity and contains less than 100 ml of clear straw coloured fluid. It is collected from and around the abdominal cavities in sterile EDTA, fluorideoxalate tube and plan tube. Fig 2.2.1 Collection of peritoneal fluid (A) Physical examination: Appearance is normally clear. Colour straw colour fluid. Normal total volume is less than 100 ml. Clot is absent. Specific gravity is less than 1.018. Clinical Pathology and Biochemistry : 14 • Clinically findings of physical examinations: 1) When Turbid: Appendicitis, Pancreatitis 2) Pale yellow: Hepatic vein obstruction, Cirrhosis, infected intestine 3) Greenish: Cholecystitis, perforated gall bladder, appendicitis ulcer 4) Milky: Parasitic infection 5) Bloody fluid: Ruptured spleen and liver, Hemorrhagic pancreatitis. (B) Chemical examination: 1) Determination of glucose: As explained in CSF Chapter by glucose oxidase method. Glucose level reduces in tuberculosis peritonitis. 2) Determinationof Amylase: • Method: By iodometric method or visible kinetic method, commercial kit is available. • Procedure: Add 1 ml of reconstituted amylase reagent and add 0.02 ml of fluid and read the absorbance every 30 seconds for 2 min. calculate mean difference and multiply by factor 7123 or by a factor provided in the kit. In different clinical conditions amylase activity is found to be more than normal blood level. • Clinical significance: In acute pancreatitis, pancreatic trauma, pancreatic pseudo cyst amylase activity in this fluid is elevated above the normal blood level. Complications of abdomen, abdominal severe pain the level increases. The indications are a) haemorrhage b) post-operative hypotension c) acute abdominal pain 2.3) Pericardial fluid: Normally pericardial sac contains about 20 – 50 ml of clear, straw colour fluid without clot. Fig 2.3.1 : Pericardial fluid Clinical Pathology and Biochemistry : 15 (A) Physical examination: The parameters studied in the physical examinations are, Volume, Appearance, Colour, Specific gravity and presence of clot. Normally 20-50 ml of fluid is present which clear, straw coloured without clot. The specific gravity is below 1.018. Clinical findings of physical examination: In the congestive heart failure and inflammation amount of pericardial fluid is found to be increase. Cloudy appearance may be associated with post- myocardial infarction, myxoedema, septic conditions .In traumatic tap blood tinged fluid is seen. Appearance of grossy bloody fluid may be caused by Bacterial pericarditis, Leaking aortic syndrome, Post myocardial infarction. (B) Microscopic examination: WBC count, RBC count, Differential count. 1) Total Leukocyte count (WBC) : • Procedure: Mix the sample. If the sample is clear do not dilute the sample. Charge the Neubauer chamber directly using the clear sample and stain. Focus under low power (10X) and adjust condenser and diaphragm for maximum visualization. Switch to high dry (45X), adjust if necessary, and count cells. For an undiluted sample, usually 4 squares are counted (1, 4, 7, and 13). Count the cells in a 4 square i.e. area counted = 4 mm2 • Calculations:= ( )× Leukocytes in pericardial fluid /cu-mm = ( ) × • In case of turbid Pleural fluid: In WBC pipette take pericardial fluid up to 0.5 marks and dilute and dilute using saline containing methylene blue up to 11 marks. Diluting fluid contains acetic acid so it cause turbidity by reacting high protein contents. Mix well and wait for 10 minutes. Load the well mixed sample on Neubauer chamber. Focus under low power (10X) and adjust condenser and diaphragm for maximum visualization. Switch to high dry (45X), adjust if necessary, and count cells. Usually 4 squares are counted (1, 4, 7, and 13). Count the cells in a 4 square i.e. area counted = 4 mm2 • Calculations: Leukocytes in diluted pleural fluid /cu-mm = × × Differentials WBC count: Centrifuge the pericardial fluid and make two smears of the sediments. Stain using Wright’s stain. Count 100 cells in consecutive oil-power fields. Report the percentage of each type of cell present. Lymphocytic effusion may be in the conditions like cirrhosis, cardiopulmonary disease, infection mononucleosis while in asthma; parasitic diseases Eosinophilic effusion is observed. (C) Chemical examination: 1) Determination of Protein: It is determined by Biuret method as explained previously. 2) Determination of Glucose: It is determined by glucose- oxidase method as explained previously Clinical Pathology and Biochemistry : 16 • Clinical significance: Cloudy appearance of the fluid is due to Septic conditions, post myocardial infarction syndrome rheumatic inflammation. Volume may increase in the conditions like congestive heart failure and inflammation. The fluid is accumulated abnormally in the conditions like increase capillary permeability due to inflammation, increase hydrostatic pressure in congestive heart failure. Eosinophilic fluid is found in asthma, pneumothorax and pulmonary infarction. 2.4 Synovial fluid: Found around the joints such as knees, hip, ankle, elbow wrist shoulder. It contains a mucopolysacchride Hyaluronic acid which acts as a binding and protective agent for the connective tissues and gives viscous nature to the fluid. Fig 2.4.1 : Synovialcavity • Collection of specimen : The fluid is collected in three sterile tubes one with EDTA, Fluoride –oxalate and plain bulbs. (A) Physical examination : The parameters studied in the physical examinations are Volume, Appearance, Colour, Specific gravity and presence of clot. Normally fluidis clear, straw coloured and viscous without clot. The specific gravity is below 1.018. 1) Viscosity test : • Synovial fluid is viscous and the viscosity is due to the presence of hyaluronic acid. The viscosity is decreased due to the breakdown of hyaluronic acid by the enzyme Hyaluronidase in inflammatory disorders. • Procedure : Drop the fluid from a syringe and note the length of the tenacious string formed use scale to measure the length. The normal fluid forms a string of at least 4 cm long if the string breaks before reaching 3 cm length the viscosity is lower than normal. Clinical Pathology and Biochemistry : 17 2) Mucin clot test : Hyaluronic acid forms compact clot in the presence of acetic acid. Low concentration of hyaluronic acid does not allow the formation of firm clot. Reagent 5% v/v acetic acid • Procedure: 1. Take 20 ml of 5% (v/v) acetic acid in a beaker 2. Add 10 ml of Synovial fluid 3. Observe the formation of clot as follows; • Firm clot • Soft clot • friable clot • No clot formation Clinical findings of physical examinations: In infected and inflammatory conditions appearance of fluid is turbid. In inflammatory disorder the enzyme Hyaluronidase activity increases in the inflammatory conditions results in breakdown of hyaluronic acid. This decreases the viscosity of fluid. (B)Microscopic examination of synovial fluid: WBC count, RBC count, Differential count 1) Total leukocyte count (WBC): Perform the entire test as describe in the above, pericardial fluid chapter. Use saline containing methylene blue as diluent for turbid specimen should be used undiluted. Normal WBC count – 50cells/cu.mm b. Differential WBC count Prepare a thin smear of the sediment and stain in the same way as described earlier. Normal fluid contains about 25% of polymorph-nuclear cells in or case these cells (above 70%) indicate bacterial arthritis. Moderate increase in neutrophils (40 to 60%) is observed in rheumatic fever, Gout tuberculosis, arthritis and in rheumatic arthritis. 2) Prepare a wet cover-slip preparation. Wet cover slip preparation of the Synovial fluid and observe first under low power objective & afterwards under high power objective. The various observations are as follows; Observations Condition Urate crystals Rhomboid calcium Pyrophosphate crystals Cholesterol crystals Gouty arthritis Pseudo-gout Rheumatoid arthritis Clinical Pathology and Biochemistry : 18 3) Perform Gram’s staining and acid fast staining on the sediment smeara explained previously. (C) Chemical examination: 1) Determination of glucose: As explained in CSF Chapter by glucose oxidase method. Protein is determined by Biuret method as explain in earlier. • Clinical significance: In inflammation and infection the appearance of this fluid is turbid. In the diagnosis of joints arthritis, gout and septic arthritis the examination is found to be very useful. In the Conditions like Pseudo –gout and Rheumatoid arthritis the different crystals are present. 2.5 Gastric juice Definition: It is a juice collected from stomach consists of 99 % water, HCl, Pepsinogen, Renin, Hemopoietic factor and Mucus. It is produce in response to a hormone gastrin, Psychic factors like sight, taste, smell and the presence of food product in the intestine. Composition: Gastric is collected from stomach and it consists of 99% water, HCL, Pepsinogen, Intrinsic factor/ Haemopoietic factor. Collection of Gastric Juice: 1. Preparation of Patient: The patient is asked to take a light early dinner previous evening and to drink a glass of milk & two charcoal biscuits or teaspoonful medicinal charcoal powder. After this except for water, he takes nothing till the test is over. Brushing the teeth is not permissible because this may give false positive test for occult blood in gastric. 2. Intubation for obtaining samples: 1. The Ryle’s tube is passed into smooth. The tube must be clean, boiled in water and lubricated with glycerine. Fetching and nauseas are contracted by spraying the throat with local anaesthesia and chilling the tube in ice water. 2. The patient is encouraged to breathe deeply through nose and to swallow persistently while the tube is slowly passed into mouth. 3. The clinician should see that tube is so placed that the tip reaches lower part of stomach and should remain in this position throughout duration of collection. 4. The fasting sample is collected. 5. Histamine 0.25 mg is injected intravenously and the time is noted. 6. Stomach contents are removed for next 2 ½ hours every 15 minutes. Clinical Pathology and Biochemistry : 19 Examinations of gastric juice: 1) Physical examination: Volume: Normally fasting content is 15-20 ml and no charcoal seen. Abnormally-charcoal seen and volume increased more than 100 ml Conditions: Hyper-secretion of gastric juice, Retention of gastric contents, Regurgitation from duodenum. Colour: • Normal-Greyish • Abnormal: Yellow-Bile • Light red: Blood ulcer of stomach • Brown: Acid Haematin (action of acid on blood) Mucus: Normal small quantity present. Abnormal: Excessive Mucus from saliva Chemical Examination: Determination of free and total acidity: Collect the sample. If the fluid is turbid, filter through the gauze. Take 1 ml sample. Add 2-3 drops of Toffer’s reagent. Titrate it against 0.1N Sodium hydroxide. End point is colour changes from, red to yellow. Note the titration reading as X ml. Continue the titration as follows. . Determination of combined acids – In the above solution add 2-3 drops of phenolphthalein Titrate with 0.1 N NaOH till colour changes from yellow to pink. Note quantity of NaOH as Y ml required. Calculations: Free acid, M eq/L = X ml (reading) × 100 Normal range is 15-45 M eq/L Total acid M eq/L = Y ml (reading) × 100 Normal range is 25-55 M eq/L Determination of occult blood: Prepare a reaction mixture by adding pinch of benzidine powder, glacial acetic acid 3-4 drops and Hydrogen peroxide 0.5 ml. Add gastric sample and observed the change in colour. If blackish green colour appears blood is present due to stomach ulcers. 3. Starch: Filtrate gastric juice +2-3 drops of iodine: Blue --Positive 4. Lactic acid: Filtrate + 1-2 drops of FeCl3 : Blue/Green - Positive 5. Bile: Filtrate + Concentrate. Nitric acid from side : Purple ring - Positive III Microscopic examination: 1. Wet count: Pus Cells – Increased in stomach infection. RBC – Increased in ulcer of stomach. Detection of cancer in stomach by cytology-pap stain of gastric washing Abnormal findings: Clinical Significance 1. Isochlorohydria: Normal free & total acid 2. Hypochlorohydria: Decrease in acidity < 3 units seen in atropic gastritis, cancer of stomach .Other disease – TB, Anaemia, Diabetes Clinical Pathology and Biochemistry : 20 3. Hyperchlorohydria: Increase in acidity > 60 units Hypertropic gastritis, Gastric ulcer Duodenal ulcer, Heavy smoker, Alcoholic 4. Achlorohydria: Complete absence of acid in any of the sample during gastric analysis when free HCl is absent. Gastric juice histamine administration is described as Histamine-fast a achlorohybria / true achlorohydria. It is seen in old age & pernicious Anaemia Indications: For diagnosis of diseases of stomach & duodenum For diagnosis of Pernicious anaemia For diagnosis of Tuberculosis in children as children may swallow sputum. For diagnosis of Cancer of stomach 2.6 Sputum: • Definition “It is saliva mixed with material coughed up from lungs, bronchi” It is expectorated material collected in lungs, branch Composition- Normally only saliva is present • Collection of specimen: 1. Patient is instructed to rinse the mouth with water to avoid contamination by food residues 2. Material is required. Expectoration brought out after an attempt of coughing and not saliva. 3. The sputum is collected directly into container. The container should be clean wide mouthed bottle (capacity 30-60 ml) with tight fitting screw-cap. 4. the most common examination of sputum is detection of T. bacilli for this early morning specimen (consisting of material collected overnight bronchi is preferred. If this proves negative sample collected over period of 24 hours is preferred). 5. When the cultural examination for pyrogenic organism or fungi is to be done sputum is collected in sterile petri-dish & examined immediately. 6. In case of cultural examination for tubercle bacilli 24 hrs sample in clean bottle is preferred. Cultural examination of any kind should be done before antibiotics drug given to the patients. 7. After the specimen has been examined, reminded it is destroyed by heat or chemical disinfectant. Procedure: Routine examination of sputum Physical examination – The parameter studied under physical examination includes Quantity, Appearance, Consistency, Colour and Odour. ChemicalExamination: PH, Occult Blood MicroscopicExamination: A wet mount or cover slip preparationObserve for the elastic fibres, charcoal, Leyden crystal, fungi, parasites. Also observe the Cellslike puscells , Epithelial cells and red blood cells. Clinical Pathology and Biochemistry : 21 Staining: 1. Leishman or Giemsa stain 2. Gram stain 3. Z-N stain 4. Pap stain • Staining: The suspicious portion is selected and with a pointed loop and thin smear is prepared on a clean glass slide. The smear meant for bacteriological examination is fixed by passing through the flame 3 to 4 times. Leishman / Giemsa stained smear for cytological study: 1. This smear is not fixed by heat (stain) itself contains fixative because it contains methanol. 2. It is stain handled exactly like the blood smear. 3. In bacterial infection, the sputum will show a large no. of polymorphs (neutrophil), macrophages (Monocytes) are seen in chronic infection. 4. The presence of large no. of eosinophils in bronchial asthma & tropical eosinophilia. Smear stained by Gram stain: 1. The sputum is always contaminated by organisms in the upper respiratory tract & mouth. 2. A mixture of organism is the usual finding. Do not carry a diagnostic significance. Smear stained by Ziehl - Neelson Method: The most important staining in the routine examination of the sputum because it shows the presence of M. tuberculosis. • Procedure: 1. Smear- Heat fix 2. Carbon Fuschin – 5 min Heat 3. Wash with water 4. 20% H2SO4 – Few seconds 5. Wash with tap water 6. Methylene blue – 1-2 minutes 7. Wash with tap water b. Concentrated Methods for M. tuberculosis: many techniques are available Principle: 1. Destroy the extraneous like mucus cells & the contaminating organism by means of chemical digestion, so that only tubercle bacilli are preserved & concentrated in a small amount of the deposit obtained from the original volume of the sputum. The most commonly used method for concentration is that of Petroff’s. • Procedure: 1. A 24 hours collection of sputum is made. 2. The sputum is mixed with an equal volume of 4% NaOH and placed in the incubator at 37ºC or 30 minutes, the container is shaken every few minutes 3. The mixture of digest is now centrifuged at 3000rpm for 30 minutes and the supernatant discarded. 4. The deposit is neutralized with 8% HCL. The acid is added drop by drop using phenol red as an indicator. The neutralized deposit is the concentrated material ready for examination. • Exfoliative cytology of sputum: This is useful method for diagnosis of bronchial & lung carcinoma. Clinical Pathology and Biochemistry : 22 1. Smears are fresh material are fixed immediately (white wet) in ethyl alcohol & stained by Papanicolaou stain. 2. The diagnosis depends upon the identification of cancer cells by their special appearance. No. Test Normal 1 Morning Sp. 2-5ml 24hrs collection 24hrs Quantity Abnormal Collection above 10 ml 2 3 Colour Clear & Colourless Consistency & Colourless, Appearance Watery Opalescence Yellow Clinical Conditions Pulmonary oedema, lung abscess, bronchitis, pulmonary haemorrhage, Advanced Pulmonary tuberculosis Pus & Epithelial cells as seen in pneumonia process Bright Red Recent haemorrhage can follow acute cardiac infarction Purulent Infection & Bloody Carcinoma of the pulmonary tuberculosis lungs Microscopic Examination • No. Test Normal Abnormal Clinical Conditions 1 Pus cells Present, Few Present, Many Presence of inflammation in the respiratory tract 2 Red Blood cells May be present, Few Present, Many Presence of inflammation in the respiratory tract 3 Curschmam’s spirals Absent Present Bronchial Asthma 4 Elastic Fibres Present Breaking down of the lung parenchyma Absent Clinical Pathology and Biochemistry : 23 • Crystal No. 1 2 3 • Test Charcoal Leydon Haematoidin Cholesterol Normal Absent Absent Absent Abnormal Present Present Present Parasites No. Test Normal Abnormal 1 E. Histolytica Absent Present 2 • Larvae of Strongyloids (stercoralis) Absent Present Clinical Conditions Amoebic abscess of lung (rare condition). Rupture of liver abscess into lungs S. stercoralis or round warm infection Differential Leucocytes count No. Test Normal Abnormal 1 Neutrophil Present, Few Increased 2 Lymphocyte Present, Few Increased • • • • • • • Clinical Conditions Bronchial Asthma Haemorrhage in the lungs, Empyema, chronic tuberculosis. Chronic lung abscess 3 4 Eosinophils Absent Erythrocytes Absent Increased Present 5 Z-n stain Present Absent Clinical Conditions Pyogenic infection Early or mild cases of tuberculosis Asthma & Eosinophilic lungs Haemorrhage of inflammatory condition Tuberculosis Questions for practice What is CSF? Write the normal composition of the CSF Write the clinical significance of the CSF Write the names of serous fluid. What is the clinical significance of synovial fluid Write About Pleural Fluid : Collection , Examination, Significance Write About Synovial Fluid : Collection , Examination, significance Clinical Pathology and Biochemistry : 24 Unit 2 : Normal and abnormal process of the body Learning Objective: • To understand the basic physiology of the body. • To know about the biochemical changes under various conditions Chapter 1 : Basic physiology and biochemistry of the body Various organs of the body maintain the homeostasis chemical environment and internal environment of the body. The basic physiological functions of the body are carried out by systems like digestive system, respiratory system, circulatory system, excretory system and reproductive system. Digestive system breaks complex organic compounds in to smaller one and absorbed as per the body need and remaining is discarded by excretory system in the form of urine and faeces. The absorbed particles are circulated in the blood .Circulatory system also circulate oxygen and carbon dioxide in the body. The heat is act as a pump. Lungs help to eliminate gaseous waste and carbon dioxide. The kidney filters the blood and removes unwanted material in the form of urine. The endocrine glands secrete the hormones also called chemical messenger and regulate physiological process of the body. The enzymes produce by different organs in the body regulate the intracellular biochemical activities of the body. Diagnostic enzymology is used to assess the function of specific organ. Body consumes inorganic compounds like salts, water, acid, bases. Inorganic salts control many physical process of the body like osmotic pressure. The organic compounds contains carbon atom as an essential constituent. They are carbohydrates, proteins, lipids, vitamins and used to synthesis various other biomolecules into the body. They have various functions like supply and storage of energy, building of body structure, regulation of interrelated activities of various organs etc. • Types of nutrients: • • Macronutrients: These are nutrients required in large quantity in food, they are large molecules, so must be digested are called as macronutrients, carbohydrate, proteins and lipids. Micronutrients: These are nutrients required in small quantity in food; they are small molecules, so they do not undergo digestion are called as micronutrients minerals,vitamins. Clinical Pathology and Biochemistry : 25 Carbohydrates These areorganic compound with elementary composition C, H and O carbon, hydrogen and oxygen. Sugars are carbohydrates and classified as monosaccharides e.g. Glucose, fructose, Disaccharides e.g. maltose sucrose and polysaccharide e.g. starch glycogen. Polysaccharides are hydrolysed to sugar. Digestion of the food in the digestive tract is a hydrolysis accomplished by various digestive enzymes. Fig. 2.1.aGlucose • Definition: They are hydrates of C, H, and O group. A) Classification: Clinical Pathology and Biochemistry : 26 1) Monosaccharide: They are simple sugars. Monosaccharides are also classified as Aldoses containing CHO group and Ketoses containing CO group as a functional groups. Depending upon the number of carbon atoms present monosaccharides are classified as follows. Monosaccharides with three carbon atoms are called trioses, those with four are called tetroses, five are called pentoses and six are hexoses, and so on. These two systems of classification are often combined. For example, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose(a six-carbon ketone). 2) Oligosaccharides: These are the carbohydrates which upon hydrolysis produce 2 to 10 units of monosaccharides. For example,sucrose, lactose, maltose are disaccharides. 3) Polysaccharide: They contain more than 10 molecules of monosaccharide. a. Homopolysaccharide: Consist of same monosaccharide units joined together. For example, .Starch, cellulose, glycogen, dextrin. b. Hetero polysaccharide: Contain different Monosaccharide units joined together. For example - Chondroitin sulphate • The general properties of these groupings are found below. 1. MONOSACCHARIDE - These are crystalline compounds, soluble in water, sweet to taste, and needs digestion in order to be absorbed into the blood stream. They may contain either five carbons (pentose) or six carbons (hexose). 2. DISACCHARIDES–Combination of two monosachhrides. These are crystalline, water-soluble, sweet to the taste, and must be digested to monosaccharides before they can be absorbed and used for energy. . 3. POLYSACCHARIDES -These are not water soluble and are not crystalline. They form colloidal suspensions instead of solutions. They are not sweet and must be digested before being absorbed. They are made up of many polysaccharides joined together. The water solubility obviously depended on the molecular weight of carbohydrates in water. Source of carbohydrates: Plants- leaves, stem, root, fruit, seeds wheat, rice, potato Clinical Pathology and Biochemistry : 27 Digestion and absorption of carbohydrates: Enzyme secretion produces sugars which are further absorbed in the body. Sr. no Organ 1 Salivary Glands 2 Pancreas 3 Small Intestine secretion Saliva Enzyme present Function of the enzyme Salivary amylase Cooked starch Maltose Pancreatic juice Amylase Invertase Invertase starch -maltose Sucrose glucose Importance of carbohydrates: 1) Carbohydrates of least expensive source of energy for body. 2) One gram of carbohydrate on oxidation yields 4 kcal of energy. 3) Glucose is the main source of energy. 4) Glucose is converted into glycogen & stored in liver & muscles. 5) Liver glycogen can be converted to glucose to maintain blood sugar level. 6) Muscle glycogen supply energy to muscles. 7) Glucose is converted to fats for energy reserve. 8) Roughage constitutes polysaccharides, to reduce gastrointestinal disorder & to make bowel movements easy. Carbohydrates : Metabolism 1) Glycolysis: • Definition: It is oxidation of glucose to pyruvate and lactate is called as glycolysis. This is called as EMP pathway (Embden-Meyerhof) pathway. Clinical Pathology and Biochemistry : 28 2) Glycogenesis: • Definition: Formation of glycogen from glucose and it is stored in liver & muscle tissue is called as glycogenesis. 3) Gluconeogenesis: • Definition: The formation of glucose from non-carbohydrate such as glycerol, pyruvic acid, lactic acid is called as gluconeogenesis. When carbohydrate reserve in body is finished, it is maintained by gluconeogenesis for energy Ex. starvation, hypoglycaemia 4) Glycogenolysis: • Definition: The breakdown of glycogen to glucose phosphate or glucose is called as glycogenolysis. 5) Glycosuria: The excretion of glucose in urine is called as glycosuria. Under normal conditions, glucose which is filtrated by glomerulus is reabsorbed in renal tubules. The maximum rate of absorption of glucose in renal tubules is 350 mg/min & is called TMG. (Tubular maximum glucose) or renal threshold (160-180 mg/100ml), rate of filtration of glucose in glomeruli is more than TMG. The excess glucose is excreted in urine is called as glycosuria. • Types: A). Alimentary Glycosuria: Definition: Glucose may appear in urine temporarily due to high intake of carbohydrate diet. But blood sugar level is normal is called as alimentary glycosuria. B). Renal Glycosuria: Definition Some defects in nephron lead to glucose excretion in urine. But blood sugar level is normal is called as renal glycosuria. Clinical Pathology and Biochemistry : 29 c) Diabetic Glycosuria: Definition Due to insulin deficiency, blood sugar level in increased and extra glucose is excreted in urine is seen in diabetes mellitus and is called as diabetic glycosuria 6) Kreb’s Cycle: This is also called as TCA or Tricarboxylic acid or citric acid cycle. It is final common pathway of oxidation of glucose, fatty acid and amino acid through which acetyl-CoA is completely oxidized to CO2 and H2O. 7) Blood Glucose Regulation Blood glucose level in blood remains 100 mg/dl during 24 hrs. A balance of these processed keeps the blood sugar level within normal limits throughout the day-average 100mg/dl . • Following ways glucose is added to blood: 1. Food absorption from intestine 2. Breakdown of liver glycogen to glucose 3. By gluconeogenesis • Following ways glucose is removed from blood: 1. By conversion to liver glycogen 2. By conversion to muscle glycogen • Following organs help for regulation f glucose level: 1. Liver 2. Muscle 3. Kidney • Following hormones help for regulation of glucose level: 1. Insulin – Decrease in Blood Sugar Level 2. Thyroxine – Increase in Blood Sugar Level 3. Glycogen - Increase in Blood Sugar Level 4. Glucocorticoid - Increase in Blood Sugar Level 5. Growth hormone - Increase in Blood Sugar Level • Insulin – Mechanism 1. Glycolysis 2. Glucogenesis 3. Decreasing glycogenolysis The body maintains a minimal level of glucose in the blood, about 70 mg/dl, and also regulates surges of glucose, when you eat a meal, to not exceed 140 mg/dl. When you are not eating, your liver has stored glucose, called liver glycogen, readily available to keep your blood levels at a minimum functioning level. Insulin is minimally at work when there is no food, but another hormone called glucagon is responsible for breaking down the glycogen stores. Your muscles also have stored glucose, muscle glycogen that is constantly being burned for energy - more so when you move. This is the “baseline of fuel” that must be maintained to keep alive. When you eat a meal, and the food is digested, your blood glucose rises. Typically, two hours after a meal is the highest concentration of glucose in the blood. This rise in blood glucose signals the pancreas to release insulin from the beta cells. Insulin makes the glucose available to the cells of the body. From the first bite of food, there is a burst of insulin secreted to control blood sugar rise. Then a steady stream of insulin is released to handle the continued digestion of the meal. Around the clock, a small amount of Clinical Pathology and Biochemistry : 30 insulin keeps control over blood glucose. Insulin’s effect is to lower your blood glucose by transporting the glucose into the cells of the body to be burned for energy or stored as fat. Another hormone, called amylin, is released with the insulin and works in the intestinal tract to regulate glucose absorption. When this complex system of fueling your body malfunctions, you develop diabetes. 8) Formation of Osazone crystals: The reducing sugars form characteristic osazone crystals. These are obtained by adding a mixture of phenyl hydrazine hydrochloride & sodium acetate to the sugar solution and then by heating the mixture in a boiling water bath. These compounds have characteristic crystal structures, melting points and precipitation times and are valuable in the identification of sugars, glucose fructose and mannose give the same types of osazones (needle shaped)and maltose forms sunflower type crystals and lactose. Forms cotton ball types of osazones. Hence lactose can be differentiated from other reducing substances i.e. present in urine. • Osazone crystals: 1. Glucose, Fructose, mannose – Needle shaped 2. Maltose – Sunflower shape 3. Lactose – Cotton ball shape Protein Fig. 2.1.b Myoglobin These are organic nitrogenous compound amino acids linked together by peptide bond and form proteins. The elementary composition is C,(carbon),H( Hydrogen), N( Nitrogen), S( Sulphur),Oxygen(O) and occasionally Phosphorus ,iron and other metals. Proteins are classified as simple and conjugated. They are basic structural component of the body; act as a secondary source of energy, upon hydrolysis produce amino acids. • Definition: • These are organic nitrogenous compound amino acids linked together by peptide bond and form proteins, polymers of amino acids & contain C, H, O,N. Clinical Pathology and Biochemistry : 31 • Source of protein: The different sources of proteins are Black eyed peas, Chick peas, Green peas, Kidney - Beans, Lentils, Peanuts, and White Beans. 1) Classification: • A) Simple protein: Ex. albumin, globulin, Fibrous protein: keratin -nail, collagen –skin, bone, elastin - ligaments. • B) Conjugated protein: They contain simple protein & non protein substance (Prosthetic group) 1) Phospho-protein: Are combination of protein with phosphorus as prosthetic Group Ex. Milk: casein, egg yolk: vitellin 2) Lipoprotein: Are combination of protein with lipids as prosthetic group Ex. Egg yolk 3) Glycoprotein: Are combination of protein with sugars as prosthetic group Ex. Mucin 4) Nucleoprotein: Are combination of protein with nucleic acid as prosthetic Group. Ex. DNA, RNA. 5) Porphyrinoprotein: Are combination of protein with porphyrin & metal as prosthetic Group. Ex. Haemoglobin • C) Derived protein: They are breakdown of proteins as a result of partial hydrolysis of proteins due to removal of prosthetic group or due to other changes in molecule. 1). Primary Derived protein: No hydrolytic cleavage of peptide bond. 2). Secondary Derived protein: Hydrolytic cleavage of peptide bonds. 2) Classification of protein Based on amino acids: 1) Complete protein: It contains all essential amino acids require maintaining normal life & growth. 2) Partially complete protein: Proteins can maintain life but not growth 3) Incomplete protein: Protein lack one or more essential amino acids. It cannot maintain life. • Functions Of protein: 1. 2. 3. 4. • Protein nutrition 5. Transport of lipids Osmotic balance. 6. Blood coagulation ( by fibrinogen ) Water balance 7. Transfer of oxygen to tissues ( by Haemoglobin) Buffering action 8. Transfer of CO 2 to tissues ( by Haemoglobin ) Digestion &Absorption: Sr.no 1 2 3 Organ Stomach Pancreas Small Intestine secretion Gastric juice, Trypsin Erepsin pepsin, HCl Function .proteins polypeptides +peptide Polypeptides/peptone Amino acids polypeptides amino acid Clinical Pathology and Biochemistry : 32 • Importance of proteins 1) Proteins are building blocks of body. 2) Proteins are essential for growth & repair of body. 3) Proteins are major constituents of bone, tooth, skin, nails, hair, and blood cells. 4) Proteins serve as source of energy. 5) DNA, RNA are proteins, present in nucleus & cytoplasm. • Properties of Proteins: 1. De-naturation of Proteins: De-naturation is when protein undergoes changes in structure of composition caused by Heat, Mineral, Acids, Alkalis, Shaking, Grinding, Alcohol, Ultraviolet rays, Ultrasonic waves. 2. Chromatography: Separation of proteins 3. Colour reactions of proteins: With biuret reagent-Purple colour complex With Bromocresol green - Greenish blue colour. 4. Electrophoresis: Movements of proteins-Albumin, 1, 2, , , globulin • Structure of Proteins: 1 Primary structure of proteins: The number and order of amino acids in polypeptide chain is referred to as primary structure of proteins. Di-peptide- 2 amino acids & one peptide linkage Tri-peptide- 3 amino acids and 2 peptide linkages Tetra-peptide- 4 amino acids & 3 peptide linkages Polypeptide- More than 10 peptides 2 Secondary structure of proteins: The folding of polypeptide chains into a specific coiled structure held together by disulphide & hydrogen bonds is refereed as secondary structure of proteins. 3 Tertiary structures of proteins: Twisted chains of protein into specific layers, crystals, fibre’s is called tertiary structure of proteins. 4 Quaternary structure of proteins: Some proteins may display a fourth level of organization where several primary, secondary, tertiary structures may combine to form quaternary (4) structure of proteins. • Nitrogen Equilibrium/ Nitrogen balance: There is constant synthesis and breakdown of protein in body. Anabolism and Catabolism of proteins are equally important and should go hand in hand with equilibrium. 1. Positive Nitrogen balance: Growth means addition of cells which in term means addition of proteins (nitrogen containing substances) in body. Retention of nitrogen in body indicates growth between ingested nitrogen in diet and nitrogen excreted in urine & faeces. Loss of nitrogen excreted in growth which shows that nitrogen is retained in body. In this case nitrogen balance is said to be positive e.g. in growth and Pregnancy Clinical Pathology and Biochemistry : 33 2. Nitrogen balance: After growth has been attained, individual should have nitrogen equilibrium. I.e. Amount of nitrogen ingested and that excreted should be equal. 3. Negative Nitrogen balance: Amount of nitrogen excreted is greater than they are formed as a result, body will lose weight, and loss of tissue proteins is negative nitrogen balance. Examples Starvation, Tuberculosis, post-operative Condition, Burn. • Protein Metabolism: • 1) De-amination: It is the process in liver that occurs during metabolism of amino acid. The amino acid (-NH2) is removed from amino acids is called as de-amination. & converted to ammonia which is ultimately converted to urea & excreted De-amination Amino acids --------------------------- Keto acid + NH3 Amino acid oxidase De-amination is carried out by amino acid oxidase. This results in the conversion of amino acid into keto acid and ammonia. Ammonia produced in liver is toxic so it is converted into urea & excreted through kidney. • 2) Trans-amination: A process involved in metabolism of amino acid in which amino group (NH2) is transferred from amino acid to keto acid with production of new keto acid & amino acid is called as trans-amination. The reaction is catalyzed by enzyme which requires pyridoxal phosphate as a co-enzyme. • 3) Urea Formation: Urea formation takes place mainly in liver. Amino acid is converted into ammonia by removal of –NH2 group. Ammonia is toxic, so it is converted into urea & excreted through kidney. This cycle is called as Urea cycle or Kerb’s-Hens kit cycle. Normal blood urea level is 15-45 mg/dl. Clinical Pathology and Biochemistry : 34 • Urea cycle: Fig.2.1 c Urea cycle • 4) Creatinine Metabolism: Creatinine is end product of creatine metabolism. Normal range of serum creatinine is 0.7-1.4 mg%. It is increased in renal failure. It is an anhydride of creatinine. Creatinine present in muscle brain & blood in free form as well as in the form of creatine phosphate. Traces of creatine are also present in urine. Creatine is largely formed in muscle by the irreversible removal of water from creatine phosphate. Formation of creatine is a preliminary step required for the excretion of most of the creatine. Amino acid, Glycine, arginine & methionine are directly involved in the synthesis of creatine. The resulting muscle contains four times more creatine phosphate than ATP. This indicates that creatine phosphate serve as the high energy phosphate store house in muscle reaction. Creatine-Phosphate + ADP = ATP + Creatine During severe exercise creatine phosphate stored in the muscle is converted to ATP. Urine excretion of creatine is externally low. It is excreted in urine, particularly in muscular disorders. Creatine formed as the end product of creatine metabolism is a waste product it is filtered at the glomeruli & secreted by the tubules & it’s excretion in urine per 24 hrs. 1.5-3.0 gm. The plasma creatine increase in renal disease, pre-renal factors which increase blood urea have little influence on blood creatine. The method commonly used for estimation of creatine makes use of Jaffe’s reaction which leads to the production of yellowish red colour with an alkaline picrate reagent. • 5) Uric acid: Uric acid is the end product of Purine metabolism, the two purine found in RNA & DNA are adenine & guanine.The first step in the catabolism of purines is their hydrolytic de-amination to form xanthine & hypoxanthine. These are then oxidized to uric acid. Clinical Pathology and Biochemistry : 35 • Gout: In this condition the characteristic features is an increase in the uric acid pool in the body. The cause of high plasma uric acid in gout is not known in all cases but it is usually due to increased endogenous synthesis of uric acid from simple precursors as inborn metabolic defect. Alcoholism & high proportion of Non-vegetarian contents in diet may be pre-disposing factor. Urates deposited in diet may be pre-disposing factors. Urates & deposits in solid form in & about the joints in the blood level is often raised serum uric acid. Determination has some diagnostic value in differentiating gout from non - gouty arthritis. The renal clearance of urate is uncharged until there is secondary renal damage due to urate deposition sometime with calculi. Clinical gout occurs mainly due to raised serum uric acid. It may occur for the following reasons; 1. Metabolic defect such as excessive breakdown of cell nuclei in leukaemia, Myelo-proliferative disease. 2. Renal causes – In renal failure or when there is obstruction to the excretion of urine, due to impaired renal function serum uric acid increase with elevated urea. Amino acids Definition: These are building block of proteins; basic formula is NH2-CH(R)-COOH. R is various organic chains, or compounds. Amino acids are biologically important organic compounds composed of amine (-NH2) and carboxylic acid (-COOH) functional groups, along with a side-chain specific to each amino acid. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen, though other elements are found in the side-chains of certain amino acids. Amino acid undergo de-amination in the liver and produce urea in the liver through ornithine cycle and remaining organic group enter in Tri carboxylic acid cycle (TCA cycle) to produce energy. Fig 2.1.d : Basic structure of amino acid Names and Sources of amino acid in food: Alanine, Histidine, Arginine, Isoleucine ,Asparagine, Lysine, Aspartic Acid Methionine, Carnitine, Ornithine, Citrulline, Phenylalanine/DLPA Cysteine, Cystine, Serine,GABA , Glutamic Tyrosine, Glycine ,Valine. Acid,Threonine, Glutamine,Tryptophan, Sources of these all amino acids are cereals, Meat, Eggs etc. Clinical Pathology and Biochemistry : 36 Glutathione, Classification: • Classification of amino acid based on nutritional status of amino acid: 1) Essential amino acids: These are those amino acids which cannot be synthesized in body to meet body need. They must be taken in diet. Ex. Methionine, Threonine, Tryptophan, Valine, Isoleucine 2) Semi essential amino acids: Are those amino acids which cannot be synthesized from other amino acids. Ex. cysteine, tyrosine 3) Non essential amino acids: Are those amino acids which can be synthesized in body. They need not be taken in diet. Ex. Glycine , Alanine , Cysteine , Aspartic acid, Glutamic acid, Proline, Hydroxyproline. • Classification of amino acid based on property of side chain: 1. Neutral amino acid: Glycine, 2. Sulphur amino acid:Cysteine, Methionine 3. Acidic amino acid:Aspartic acid, Glutamic acid 4. Basic amino acid: Alanine 5. Aromatic amino acid: Tyrosine • Importance: The 20 major amino acids, plus hundreds of minor amino acids keep us alive, vibrant, and healthy. A deficiency in a single amino acid will cause problems for us. 1. Amino acids must be supplied in diet to maintain normal growth,& nitrogen balance & health. 2. Asphartic acid, Glutamic acid are involved in transmission of impulses in nervous system 3. Many Hormones are Amino acids – Adrenaline, Thyroxin and T3 4. Glycine is required for synthesis of Haem. 5. Amino acids are required for synthesis of nucleotides 6. Abnormalities in transport of Amino acids in cell results in disease • Main Functions: Most of the amino acids are important source of energy for muscle. Alanine is the primary amino acid in sugar metabolism it also boosts immune system by producing antibodies. Alanine is a major part of connective tissue. Arginine is essential for normal immune system activity, necessary for wound healing, Assists with regeneration of damaged liver, Necessary for production and release of growth hormone. Arginine is the most potent amino acid in releasing insulin and is necessary for spermatogenesis. Clinical Pathology and Biochemistry : 37 Lipids Lipids are insoluble in water soluble in organic solvents such as alcohol, ether etc. are esters of fatty acids with the basic elementary composition C,H and O. Have more hydrogen and thus can store more energy. Triglycerides are simple lipids formed by the union of glycerol and three fatty acids stearic, oleic and palmitic acid. Complex lipids contains additional chemical group like glucose, phosphate, protein. Sterols chemically related to steroids have a characteristic chemical structure .Cholesterol is important sterol of the body. Ketone bodies are acetone, aceto-acetic acid and beta hydroxyl-butyric acid. Fig.2.1.e Lipids Lipids (fats): • Definition: They are organic compound of combination of fatty acids + glycerol. • Source: Many foods contain lipids. Meats, poultry, shellfish, fish, most dairy, egg yolks and even some vegetables like avocados are all sources of lipids. Even whole grains contain some lipid. • Classification: • A) Simple lipid: They are esters of fatty acids + glycerol. Again classified as, 1) Fat: These are esters of fatty acid + glycerol Example .Tripalmitin, coconut fat, animal fat 2) Wax: These are esters of fatty acids with long chain mono-hydroxy alcohols. Example. True wax , Vitamin D esters Clinical Pathology and Biochemistry : 38 B) Compound or conjugated lipids: They are esters of fatty acids + glycerol & other compound like phosphoric acid. 1) Phospholipids: They are esters of fatty acids + glycerol & other compound like phosphoric acid. Present in nervous tissue, brain, liver and kidney. They are further classified as phosphoglycerides, phosphoinositides and phosphospingosides. Some examples of phospholipids are Cephalin, cardiolipin, plasmalogen. • Importance of phospholipids 1. To form structure of mitochondria, myelin sheath. 2. To help in coagulation Ex. Cephalin. 3. To act as prosthetic group to enzymes. 4. To form structure of mitochondria – cardiolipin. 2) Glycolipids: They are esters of fatty acids + glycerol & other compound like carbohydrate. These are further classified as Cerebrosides, Gangliosides, sulphstides, sulpholipids • • C) Derived lipid: Hydrolysis of Simple & Compound lipid produces derived lipids. It includes Fatty acid, alcohols, monoglycerides, diglycerides, steroids, terpens and carotenoids. 1) Fatty acids: These are long hydrocarbon chain. Again classified as, Saturated fatty acid which contains C-C single bond. For Example Butyric acid, Palmetic acid, Steric acid. Unsaturated fatty acids which contains double bond. They are oils at room temperature. For example Oleicacid, Linolenic acid, Linoleic acid, Arachidonic acid 2) Steroids: Common structure of Steroids nucleus consist of 3, 6-membered carbon rings &1, 5-membered carbon ring. • Importance of steroids: 1. Naturally present: Male sex hormones: TestosteronFemale sex hormones: Oestrogen, Progesterone • Importance of lipids: Triglycerides are also called blood and body fat. As body fat, triglycerides play a role in energy storage. They also provide a layer of insulation under the skin and protective cushioning around the organs. Your body also uses triglycerides to make the myelin sheaths that surround nerve cells. Myelin sheaths act as insulation and help the nerve signal travel faster along the length of the nerve. Triglycerides are solid at body temperature and are classified as saturated fats. If you have too much triglyceride in your blood, it can collect on the blood vessel walls and cause heart disease.Cholesterol is another type of blood fat. Your body uses cholesterol to make steroids such as estrogen, progesterone and testosterone. Your body also makes its own supply of vitamin D and uses cholesterol for that process. • Different functions of lipids: 1) 1gm fat gives 9 kcal of energy. 2) They act as insulators & Protect organs & body. 3) Fats act as shock absorber. 4) Fats are store form of energy. 5) They are important component of cell membrane. 6) They form Hormones 7) They are important component of nervous tissue. 8) They are important component of mitochondria. Clinical Pathology and Biochemistry : 39 9) Vitamins A, D, E and K are fat soluble vitamins and stored in the body. • Digestion and absorption of lipids: Secretion Sr No Organ 1 Pancreaase Lipase . Liver Bile ,Bile 2 salts Function Fats Fatty acids +Glycerol Digestion, absorption of fats • Atherosclerosis: Excess intake of saturated fats, causes deposition of fat in coronary artery of heart, is called as atherosclerosis. This deposit causes block to blood flow to heart & causes heart attack. • Metabolism of lipids: Fat ingested or obtained by from fat storage organ like adipose tissue is lypolysed to fatty acid and glycerol. All fatty acids are broken down to acetyl Co-A having just 2 carbon atoms. This process is called as -Oxidation of fatty acids. • – Oxidation: This is also called as metabolism of fat. Definition: Fat is broken into fatty acid & glycerol. All fatty acids are broken down to acetyl Co enzymeA& then to CO2& H2O by citric acid cycle is called as B-Oxidation of fat. Fatty acid | Active fatty acids | -Hydroxyl acetyl Co-A | aceto-acetyl Co-A | Acetyl Co-A + Acetyl Co-A | Citric acid cycle | CO2 + H2O • Lipoproteins: They are esters of fatty acids + glycerol & other compound like lipids. For example High density lipoprotein, Low density lipoprotein and Very low density lipoprotein. Clinical Pathology and Biochemistry : 40 Enzymes • Definition: They are synthesized by cells of all living organisms. They act like catalyst. &accelerate multitude of metabolic reactions upon which life depends. Enzymes are proteins & their catalytic activity depends on structure of polypeptide chains. . Enzymes are proteins used by the body to increase or decrease the speed of chemical reactions. Though there are many different kinds of enzymes, we commonly think of the digestive enzymes because they make it possible for our body to break down and assimilate the foods we eat. A diet rich in enzymes can increase energy and stamina, as well as support weight loss, healthy skin, and overall good health. • Sources of some enzymes: Papayas are a tropical fruit. They contain large amounts of the enzyme known as papain. Raw pineapples contain the enzyme brome-lain. Sprouts are the seeds of many different types of grains. They are packed with nutrients and may contain more than 100 times more enzymes than fruits and vegetable. Most of the enzymes requires in the body for different processes are synthesized in the body. • Classification: 1) Oxidoreductases: Catalyse Oxidation – reduction reaction which yields energy. For example Alcohol Dehydrogenase. 2) Transferases: Carry out transfer reaction in which one group is transferred to another .Glutamate oxaloacetate transferase. 3) Hydrolases: Catalyse hydrolytic reaction. For Example - Amylase, Lipase 4) Lyses:Catalyse reaction resulting in elimination of group Ex. Maltase, Lactase, Sucrase 5) Isomerases: Catalyse isomerisation reaction which brings about intra-molecular rearrangement in molecule .Ex. L- alkaline isomerase. 6) Ligases: Catalyse joining reaction which link two molecules. • Other Classification: 1) Functional Enzymes: These enzymes are present at all times in circulation. Perform physiological functions in blood. For example: Enzymes of blood coagulation – Thrombokinase, lipoprotein- lipase 2)Non-Functional Enzymes: Perform no physiological functions in blood. For example: Enzymes of exocrine secretion, Intracellular enzymes • Importance of Enzymes Enzymes are the sparks that start the essential chemical reactions our bodies need to live. They are necessary for digesting food, for stimulating the brain, for providing cellular energy, and for repairing all tissues, organs, and cells. There are three types of enzymes: metabolic enzymes, digestive enzymes, and food enzymes. Metabolic enzymes catalyze, or spark, the reactions within the cells. The body's organs, tissues, and cells are run by metabolic enzymes. Without them our bodies would not work. Among their chores are helping to turn phosphorus into bone, attaching iron to our red blood cells, healing wounds, thinking, and making a heart beat. Clinical Pathology and Biochemistry : 41 Digestive enzymes break down foods, allowing their nutrients to be absorbed into the bloodstream and used in body functions. Digestive enzymes ensure that we get the greatest possible nutritional value from foods. Food enzymes are enzymes supplied to us through the foods we eat. Nature has placed them there to aid in our digestion of foods. This way, we do not use as many of the body's "in-house" enzymes in the digestive process. • Diagnostic enzymes : 1. 2. 3. 4. Asses severity of organ damage Follow trend of disease Differentiate particular type of disease Determine postoperative risk. Enzyme 1. Amylase 2. SGPT , SGOT 3. CPK – MB , SGOT , LDH 4. ALP 5. Acid Phosphatase 6. Lipase Example of Condition Acute pancreatitis , Pancreatic cancer Infective hepatitis Myocardial infarction Bone disease , rickets , Obstructive jaundice Prostatic cancer Pancreatitis • Enzymes specificity: A particular Enzymes acts only on particular chemical group • Factors which affect Enzymes activity: 1. Substrate concentration 2. Enzymes concentration 3. Temperature –370C 4. PH : 5 – 9.0 5. Ionic strength. 6. Oxidation – reduction potential Clinical Pathology and Biochemistry : 42 Co-enzyme • Definition: Many enzymes catalyse reactions of their substrate only in presence of particular non-protein, loosely bound organic or inorganic compound called Co-enzyme. • Classifications: 1) Metabolic coenzyme: synthesize from common metabolite. For example. Adenine triphosphate, UDP glucose .Coenzyme A 2) Vitamin derived coenzyme: Synthesize from vitamins. For example Vitamin B-6, Thiamine. • Functions of Co-enzyme: 1. Acts as acceptor or donor of specific group of substrate. 2. Enzyme first binds to Co-enzyme molecule followed by enzymatic transfer of group &substrate binding to form product. Iso-enzyme • Definition: These are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These are physically different forms of same enzymes having same catalytic activity called Iso-enzyme. The enzyme Lactate Dehydrogenase is made of two(H-form and M-Form) different sub units, combines in different Permutations and Combinations in depending on the tissue in which it is present as shown in below table, • Examples: Creatinine phosphokinase : CK-MM : skeletal muscles, CK-BB: Brain CK-MB :Heart Alkaline phosphatase: ALP-1: Liver, ALP-2: Bone, ALP-3: Small intestine, kidney, and placenta. Type Composition Location LDH1 HHHH Heart and Erythrocytes LDH2 HHHM Heart and Erythrocytes LDH3 HHMM Brain and Kidney LDH4 HMMM Skeletal Muscle and Liver LDH5 MMMM Skeletal Muscle and Liver Clinical Pathology and Biochemistry : 43 • Characteristics: 1. They catalyse same reaction. 2. They are different in physical , biochemical , immunological properties 3. Duo to difference in their molecular weight &size, they can be separated in electrophoresis. Minerals: They are essential in small quantity for different body activities. Mineral Source Sodium Calcium Table salt, vegetables. Milk, vegetables. Formation of blood. Bone, teeth Clotting blood. Potassium Iodine Iron Green leafy vegetables. Iodized salt Green leafy vegetable, Meat. Growth, Blood formation Tyroxine formation. Haemoglobin formation. Phosphorus Milk, vegetables. Minerals Functions Normal range Calcium 8.4-10.4 mg% 1. Normal Functions of body 2. Imp. Const. in bones & teeth 3. Nerve function 4. Muscle contraction 5. Blood clotting Phosphorus Adult 2.5-4.5 mg% Child:4-6 mg% Sodium 135.0155 mm d/ l 1. Important constituent of bone 2.Formation of ATP(Adenosine Tri-Phosphate) Potassium 3.55.5 mm d/ l Iodine Iron Importance 1. Extracellular fluid control 2. Acid-Base balance 3. Nerve Function 4. Muscle Function 1. Intracellular fluid control 2. Acid-base balance 1. Growth & development of body 2. Thyroid gland requires iodine for synthesis of thyroid hormones T3 & T4 1. Haemoglobin formation 2. Stored in Liver Bones teeth and ATP formation Disease condition Overdose| 1. Rickets 2. Osteoporosis 3. Osteomalacia 4. Tetany 5. Increase clotting time 1. Osteoporosis Calculi in Gall bladder/Kidney Hyponatremia 1. Diarrhoea 2. Vomiting 3. Dehydration Hypokalaemia 1. Dehydration 1. Low Iodine Low T3 T4 Hypothyroidism Hypernatremia Iron deficiency anaemia Clinical Pathology and Biochemistry : 44 Hyperkalaemia Haemosiclerosis Vitamins • Definition: A vitamin is an organic compound required by an organism as a vital nutrient in limited amounts. An organic chemical compound (or related set of compounds) is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. • Sources: Fresh fruits and vegetables • Classification: 1) Fat soluble vitamins - Viitamin A, D, E and K 2) Water soluble vitamins - B complex, vitamin C. No. Vitamin Source Function Deficiency effect 1 Vitamin A Vegetables, carrot, Tomato, milk, fish Growth vision at night 2 Vitamin D Sunlight ,milk, Fish, egg 3 Vitamin E Milk, fish, egg, Meat Leafy vegetables Ca &p absorption. Teeth & bone formation. Normal cell development, Reproduction. Poor Growth night blindness Dryness of eyes. Rickets. Osteomalacia in adult. Badly formed teeth Gums swelled. 4 Vitamin k green vegetables Cabbage eggs Blood clotting Increased clotting time. 5 Vitamin C Citrus fruits, green vegetable Development of teeth gums, bones Scurvy, swollen gums. B- Complex vitamins 5 B1 (thiamine) Co- enzyme Milk, nuts, meat, Cheese, Peas, cereals, Nerve function Vegetables Beriberi –loss of Normal appetite 6 B2 Riboflavin Milk, nuts, meat, Cheese Co-enzyme Tongue and skin infection 7 B6 Cheese, meat products Pyridoxine, help in RBC formation Nervous disorder 8 B12 Meat product cheese RBC Growth and maturation Coenzyme cyanocobalamine anaemia 9 Folic acid Meat product, cheese RBC growth and etc maturation Clinical Pathology and Biochemistry : 45 Anaemia • Importance of Vitamins: They are not synthesized in body, so they must be taken in diet. Vitamins are required for metabolism. • Functions of vitamins: Vitamins have diverse biochemical functions. Some, such as vitamin D, have hormone-like functions as regulators of mineral metabolism, or regulators of cell and tissue growth and differentiation (such as some forms of vitamin A). Others function as antioxidants (e.g., vitamin E and sometimes vitamin C). The largest number of vitamins B complex vitamins function as precursors for enzyme cofactors, that help enzymes in their work as catalysts in metabolism. In this role, vitamins may be tightly bound to enzymes as part of prosthetic groups • Water: 1 )Water is essential for life. 2) Water makes up to 50 –65% of weight of human. 3) Human adults require 1.5 litres of water /day. 4) Water is main component in blood. 5) Water acts as lubricant for joints & digestive system. 6) Water cools body by evaporation of sweat. 7) Water is useful in different metabolism. • Balance diet: • Definition: It is the diet which provides correct levels of all nutrients for Physiological & biochemical needs of body is called as balance diet. Ideal Requirements of balance diet: • 50-60% carbohydrates • 15-20% proteins • 20-30% fats • Plenty of vegetable & fruits. • Use of moderate quantity of salt. • Adequate quantity of water. • Importance of balance diet: 1) It is essential for normal function of body parts. 2) It provides nutrient for growth & development of body parts. 3) It helps to store energy in body. 4) It is important for disease resistance of body. 5) It is important for good health. • Imbalance diet: Definition: When some of the nutrients are not taken in correct quantity & proportion, is called as imbalance diet. Clinical Pathology and Biochemistry : 46 Types: Malnutrition: Imbalance of nutrition is called as malnutrition. It can be a deficiency or excess. 1) Under- nutrition: It is deficiency of calories or nutrients is called as under –nutrition. 2) Over nutrition: It is excess of calories or nutrients is called as over-nutrition. Name | Deficiency Excess Carbohydrate Retardation of body growth. Excess sugar leads to Diabetic Melllitus Proteins Retardation of body growth. Disease –marasmus & kwashiorkor Fats Retardation of body growth. Atherosclerosis. obesity Salts Retardation of body growth Hypertension. Nucleic acid • Definition: These are polymers of nucleotides. Nucleotides are joined together by phosphate diester linkage. DNA : Found mainly in nucleus of cell It is double stranded. Structure : Nitrogen bases Purine : Adenine and Guanine Pyrimidine: Cytosine, Thymine Sugar:Deoxy-Ribose Function : Transmission of genetic information to organism Control of synthesis of proteins. Control of all biochemical reactions. RNA : Found mainly in cytoplasm of cell It is single stranded. Structure : Nitrogen bases Purine present are Adenine and Guanine Pyrimidine present are Cytosine and .Uracil Sugar : D-Ribose Function: It takes genetic message from DNA& helps synthesis of proteins in cell. • Structure of DNA: • A nucleotide: It is composed of a nucleobase (also termed a nitrogenous (either riboseor deoxyribose), and one or more phosphate groups. base), a five-carbon sugar • A nucleoside: It consists simply of a nucleobase and a 5-carbon sugar. In a nucleoside, the base is bound to either ribose or deoxyribose via a beta-glycosidic linkage. Examples of nucleosides include cytidine, uridine, adenosine, guanosine, thymidine and inosine. Clinical Pathology and Biochemistry : 47 • Deoxyribonucleic acid (DNA) It is a molecule that encodes the genetic instructions used in the development and functioning of all known living organisms and many viruses. DNA is a nucleic acid. DNA molecules consist of two polypeptide chains , running in opposite direction & coiled in such a way that adenine of one strand is infront of thymine in other chain & guanine of one strand is infront of cytosine in other chain .( A : T , G : C ) .They are bounded by weak hydrogen bonds. This arrangement of bases is called as base pairing (3-5 phospho-diester linkage).This structure is known as double helical structure of DNA. In mammalian cells DNA is always found in nucleus where it exists in combination with protein giving rise to structure called chromosome. • Importance of DNA: 1) Transmission of genetic information to organism . 2) Control of synthesis of proteins 3) Control of all biochemical reactions If DNA undergoes mutation, this will affect metabolism. It leads to inborn errors of metabolism. • RNA(Ribonucleic acid) : Ribonucleic acid (RNA) is a family of large biological molecules that perform multiple vital roles in the coding, decoding, regulation, and expression of genes. RNA comprises the nucleic acids, which, along with proteins, constitute the three major macromolecules essential for all known forms of life. RNA is assembled as a chain of nucleotides, but is usually single-stranded. Cellular organisms use messenger RNA (mRNA) to convey genetic information (often notated using the letters G, A, U, and C for the nucleotides guanine, adenine, uracil and cytosine) that directs synthesis of specific proteins, while many viruses encode their genetic information using an RNA genome. • Types of RNA: 1. M-RNA: Messenger RNA: It is single stranded of low molecular weight which is synthesized in nucleus of cell. The sequence of Nucleotides in RNA determines by base pairing rule that one strand of RNA is complementary to other strand of DNA. Only difference is replacement of thymine to uracil in RNA. This process is known as Transcription of genetic information. 2. r-RNA: Ribosomal RNA: It is single stranded of low molecular weight, associated with ribosomes. Function: To maintain structural integrity of ribosomes & to bind m-RNA & t-RNA in synthesis of proteins. Clinical Pathology and Biochemistry : 48 3. t-RNA: Transfer RNA: It is single stranded of low molecular weight. Function: Transfer of amino acids to synthesis of proteins. For each amino acid, there is specific transfer RNA Diagram: Clinical Pathology and Biochemistry : 49 Bilirubin: It is a pigmented non protein nitrogenous compound originates from the breakdown of haemoglobin. Haemoglobin consists of haem (iron and Protoporphyrin a non-iron pigment) and protein globin. It degraded in reticuloendothelial system. Protoporphyrin produce free bilirubin which is insoluble in water transported to liver and gets conjugated to glucuronic acid to produce conjugated bilirubin. It is water soluble, excreted from the liver into the duodenum through bile. In the intestine it is converted to urobilinogen and excreted through faeces. RNA (ribo-nucleic-acid), DNA (deoxyribonucleic acid), nitrogenous compound DNA carrier of genetic information. Consist of purines and pyrimidine. Adenine and Guanine are the purines uracil, cytosine, thymine are pyrimidine. Nucleotides are structural units of nucleic acid composed of phosphoric acid, sugar and nitrogen base. ADP and ATP are adenosine di-phosphate and adenosine tri-phosphate respectively and participate in the energy transfer process of the body. Questions for practice : • Define, classify and importance of carbohydrates • Define, classify and importance of proteins • Define, classify and importance of lipids • Define, classify and importance of nucleic acid • Define ,classify and importance of amino acids • Define, classify and importance of enzymes Clinical Pathology and Biochemistry : 50 Chapter 2 : Interrelated metabolic processes of body Inter-related metabolic processes of body: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. The numbers of processes are going on in the body. Synthetic i.e. anabolic and degradative i.e. catabolic processes. Together it is termed as metabolic processes that are closely interconnected. For the synthesis of complex organic compounds from simpler compounds, energy is required, energy is consumed in the body for synthesis. Many biomolecules are synthesized in the body. Some of the important synthetic reactions of the body are formation of glycogen from glucose proteins from amino acids, fats from fatty acids, & glycerol and urea from ammonia & carbon dioxide. Conversely, most of the degradation processes yield simpler products from complex organic compounds. Digestion and Oxidation are the two most common degradation processes. Simpler products of digestion are oxidized aerobically (oxygen compounds) to the ultimate molecules of water and carbon dioxide. Energy released by the breakdown of chemical bonds is available for the growth and development of the body. Mitochondria, present in cells are the seat of Kreb’s cycle. Dietary glucose is stored by the liver as glycogen (animal starch) which is a polysaccharide. When the body needs energy, glycogen is first converted into glucose. In the following biochemical steps, glucose is anaerobically degraded. ( the process is called glycolysis & does not require oxygen) to pyruvic acid (3-C compound) Glucose-6 phosphate is one of the intermediate products of glycolysis which is capable of supplying energy to red cells without the aid of oxygen; the metabolic pathway belongs to the hexose monophosphate shunt. Pyruvic acid is the end product of glycolysis that may either lead to the formation of lactic acid under anaerobic conditions or may lose one carbon, forming acetate (2-c compounds, acetyl- Coenzyme A ) prior to its entry into the Kreb’s cycle. The acetate thus acts as the junction box for the metabolic products of carbohydrates, fats & proteins in order to supply energy for the growth and development of the body. At the final stage of oxidation, the acetate enters the Kreb’s cycle and oxidatively ‘burns out” with the intake of the oxygen and releasing carbon dioxide, water molecules and energy as the end products. Question for practice ; • write about interrelated metabolic processes of body Clinical Pathology and Biochemistry : 51 Chapter 3 : Functions of various organs and their clinical assessment • Liver: Liver synthesizes albumin, fibrinogen, urea, uric acid, prothrombin, lipoprotein, transferrin, glycoprotein, hipuuric acid cholesterol and other lipids. Glucose is stored in the liver as glycogen. It also store fat soluble vitamins and vitamin B Excretory functions of liver consist of removal of bilirubin, detoxification function synthesis of urea from toxic ammonia. Jaundice, hepatitis, cirrhosis, fatty liver, infection, amyloidal conditions are some pathogenic conditions of the liver. • Kidney: Filtration of blood, re-absorption of essential ingredients from the filtrate and urine formation, maintains homeostasis of the body, body temperature, electrolyte balance. • Heart: It is centre of the circulatory system. It acts like a muscular pump which helps in blood circulation. To assess the proper functioning of the heart the creatinine phosphokinase, Lactate dehydrogenase, serum glutamate oxaloacetate transferase enzymes are used. • Pancreas: The external secretions are amylase and lipase digestive enzymes. The internal secretions are insulin and glucagon .Insulin is endocrine hormone secreted by beta cells of islet of Langerhans. • Endocrine glands: They are ductless glands which produce internal secretion or hormones which act as chemical messengers. Hormones are mostly steroids, proteins and amines; regulate the interdependent metabolic process of the body and its development. Insulin plays an important role in the carbohydrate metabolism and its deficiency leads to hyperglycaemia and glycosuria, Thyroid hormones regulate many metabolic processes are tri-iodothyronine T3 and thyroxin T4. Pituitary, brain and hypothalamus triggers and control the secretion of hormones. Pituitary consists of anterior and posterior lob. Anterior secrete TSH, ACTH, Gonadotropins while posterior secretes ADH. The pituitary independent hormones are insulin, serotonin etc. • Lungs: Gaseous exchange supply oxygen to cells and remove carbon dioxide produce during metabolic process. Haemoglobin acts as a carrier of these gases. Respiratory acidosis and alkalosis is due to fluctuation of the partial pressure of the carbon dioxide in the blood. • Brain: It is a primary centre for regulating and coordinating various activities of the body. CSF connects brain with spinal cord. Questions for practice : • write about Functions of various organs & their clinical assessment: Clinical Pathology and Biochemistry : 52 Chapter 4 : Biochemical changes in the body under pathologic conditions Homeostasis is maintained by body and if disturbed pathological state is suspected. Most organs have specific enzyme disturbance in that organ increases the release of that enzyme in the blood stream and it may be the pathological state of that organ. To access these functions routine test profile and diagnostic biochemical test profile are used. The profile contains group of test are called as a diagnostic biochemical test profile. • Kidney function panel: To maintain the homeostasis, waste product of creatine phosphate a creatinine is excreted by the kidney. The normal range of creatinine is 0.7 to 1.4 mg/dl. Amino acids are converted to urea in the liver and urea is excreted by kidney .The normal range is 8 to 40 mg/dl. The uric acid is formed from the breakdown of nucleic acid and excreted by the kidney. The normal range of Uric acid is 3.5 to 7.5 mg/dl. When renal function is abnormal the values of Uric acid, Urea and Creatinine are increases in the blood. So these three tests are kidney panel test. • Liver function test: Liver is the Site of metabolism, synthesis of proteins like albumins, lipoproteins, transport protein, cholesterol, fibrinogen and bilirubin. Mostly Bilirubin, transaminases enzymes (SGPT, SGOT) Proteins, albumin, globulin A/G ratio is used as liver panel tests. Bilirubin is waste product from the breakdown of haemoglobin forms in the liver as a conjugated bilirubin, conjugated to glucuronic acid and excreted into the bile. Conjugated bilirubin is also called as direct bilirubin. The normal range of total bilirubin is 0.1 to 1.2 mg/dl and of direct bilirubin it is 0 to 0.6 mg//dl. Various metabolic activities of liver are accomplished by enzymes like Alkaline phosphate, normal range 20 is 130 U/L, Lactate de-hydrogenase (LDH) the normal range is 110 to 239 U/L, SGPT Also called ALT i.e. serum glutamate pyruvate transaminase the normal range is 3 to 30 U/L, SGOT i.e. serum glutamate oxaloacetate transaminase the normal range is 10 to 37 U/L also called AST., Gamma glutamyl transferase GGT the normal range is 3 to 40 U/l . During liver cell destruction, cell injury, damage, tumours, lesions, hepatitis infection the enzyme concentration in the blood increases. • Cardiac panel: It includes AST, LDH and Creatine phosphokinase. The normal range of CPK is 30 to 170 U/L and increase in the value is related to injury to the cardiac muscles. • Lipid profile: Lipids are closely associated to the cardiovascular diseases. These include cholesterol, HDL, LDL, VLDL, Triglycerides. Cholesterol normal range is 200 mg/dl and elevated levels are the risk of coronary artery diseases. Triglycerides are major form of neutral lipid. Clinical Pathology and Biochemistry : 53 • Thyroid function test: Thyroid gland synthesizes T3triiodothyronine and T4 Thyroxin from amino acid tyrosine and iodine under the influence of TSH thyroid stimulating hormone. Hyperthyroidism increase secretion thyroid gland which happens in Grave’s disease and insufficient secretion happens in Myxoedema are the pathological states of thyroid secretions. • Mineral metabolism: The important minerals are sodium, potassium, calcium, phosphate, iron. Calcium and phosphorus are needed for proper bone and teeth development. Normal range for calcium is 8.7 to 10.5 mg/dl and for phosphorus it is 3 to 4.5 mg/dl. Iron is needed for Haemoglobin synthesis whose normal range is 65to165 micromoles/l. In the blood the protein transferrin is used to transport the iron. Iron deficiency leads to anaemia. Sodium is a chief cation of extracellular fluid and potassium is a chief cation of intracellular fluid. Questions for practice • Write the function of heart and lungs • What is the function of calcium and phosphorus? Clinical Pathology and Biochemistry : 54 Unit 3 : Routine Biochemical Tests Learning Objectives: We can learn about the enzymes like • Transaminases • Phosphatases • Lactate dehydrogenase • Creatnine kinase • Electrolytes • Blood gases • Determination of s. biocarbonate Chapter 1 : Transaminases • Definition: These are the enzymes which are also called as amino-transferase. They catalyses a type of reaction between an amino acid and a- -keto -acid. An amino acid contains an amine (NH2) group. A keto acid contains a keto (=O) group. • Function: In transamination, the NH2 group on one molecule is exchanged with the =O group on the other molecule. The amino acid becomes a keto acid, and the keto acid becomes an amino acid. • Importance of Transaminases: The transaminase enzymes are important in the production of various amino acids. The measurement of the concentrations of various transaminases in the blood is important in the diagnosis of many diseases. Many transamination reactions occur in tissues, catalysed by transaminases specific for a particular amino/ keto acid pair. The reactions are readily reversible. Two important transaminase enzymes are AST (SGOT) and ALT (SGPT), the presence of elevated transaminases can be an indicator of liver damage. The preference of liver transaminase for oxalo-acetate or alpha-keto-glutamate plays a key role in Clinical Pathology and Biochemistry : 55 funnelling nitrogen from amino acid metabolism to aspartate and glutamate for conversion to urea, for excretion of nitrogen. In muscles the pyruvate is used for transamination and gives alanine, which is carried by the bloodstream to the liver. Here other transaminases regenerate pyruvate, which provides a valuable precursor for gluconeogenesis i.e. synthesis of glucose from non-carbohydrates source. • Determination of serum glutamate pyruvate amino-transferase (SGPT/ALT) and serum glutamate oxalo-acetate amino-transferase (SGOT/AST): • Clinical significance: ALT is found predominantly in the liver, with clinically negligible quantities found in the kidneys, heart, and skeletal muscle, while AST is found in the liver, heart (cardiac muscle), skeletal muscle, kidneys, brain, and red blood cells. As a result, ALT is a more specific indicator of liver inflammation, liver damage, liver cirrhosis the disease conditions associated with the liver. AST may be elevated also in diseases affecting other organs, such as myocardial infarction, acute pancreatitis, acute haemolytic anaemia, severe burns, acute renal disease, musculoskeletal diseases, and trauma. AST was defined as a biochemical marker for the diagnosis of acute myocardial infarction. • Method: End point Reitman and the Frankel’s method. Enzyme unit: Karmen units: These are expressed as the units of the activity which produces change in the O.D. of 0.001per minute by enzyme present in the 1 ml of the serum. • Principle: The following reaction is catalysed by the enzyme glutamate oxalo-acetate transaminases (SGOT).This enzyme catalysed the reversible transfer of amino group from glutamate to oxalo-acetate simultaneously replacing the amino group of glutamate to carbonyl group and gets converted to aspartate. In the Reitman-Frankel reaction the SGOT catalyzes the reaction of aspartic acid and alpha-ketoglutaric acid to oxalo-acetic acid and glutamic acid. The oxalo-acetic acid is treated with 2,4dinitrophenylhydrazine in an alkaline medium to produce a highly colored hydrazine which can be measured calorimetrically. The following reaction is catalysed by the enzyme glutamate amino-transferase( SGPT).This enzyme catalysed the reversible transfer of amino group from glutamate to pyruvate simultaneously replacing the amino group of glutamate to carbonyl group and get converted to alanine. Clinical Pathology and Biochemistry : 56 SGPT workson a substrate.. The pyruvic acid is treated with 2, 4-dinitrophenylhydrazine in an alkaline medium to form a highly coloured hydrazine which is measured photo-metrically. • Reagents: SGPT substrate: It contains 1.78 gram of alanine, 30 mg of alpha ketoglutaric acid 0.5 ml of 1 N NaOH in a phosphate buffer of pH 7.45.Make the final volume 100 ml using buffer. SGOT substrate: IT contains 2.66 gram of Aspartic acid, 30 mg of alpha ketoglutaric acid 20 ml of 1 N NaOH in a phosphate buffer of pH 7.45.Make the final volume 100 ml using buffer. DNPH reagent: 200 mg of di-nitro-phenyl hydrazine in 0.01 N HCL 0.4 N Sodium Hydroxide., 22 mg/dl Sodium pyruvate Stability of the reagent: The reagents are stable at 2oC to 80C. • Sample: Un-hemolysed serum sample • Procedure: Preparation of calibration graph for SGPT: 1 2 3 0.45 0.4 0.35 0.3 0.5 Standard Sodium Pyruvate in ml 0.05 0.1 0.15 0.2 - Distilled water in ml 0.1 0.1 0.1 0.1 0.1 DNPH in ml 0.5 0.5 0.5 0.5 0.5 Mix and keep for 20 min at room Temp. 0.4 N NaOH in ml 5 5 5 5 5 Karmen Units 28 57 97 150 0 SGPT substrate in ml Clinical Pathology and Biochemistry : 57 4 Blank • Protocol for SGPT determination: Test Blank SGPT Substrate in 0.5 0.5 ml Incubate at 370C For 5 min Serum in ml 0.1 Incubate at 370C For 30min DNPH in ml 0.5 o.5 Serum in ml 0.1 Mix and keep At Room For 20 minutes Temp. 0.4 N NaOH 5 5 Mix and keep at room temperature for 10 min. Read the intensity of the test against blank at 540 nm. • Preparation of calibration graph for SGOT: 1 2 3 0.45 0.4 0.35 0.3 0.5 Standard Sodium Pyruvate in ml 0.05 0.1 0.15 0.2 - Distilled water in ml 0.1 0.1 0.1 0.1 0.1 DNPH in ml 0.5 0.5 0.5 0.5 0.5 Mix and keep for 20 min at room Temp. 0.4 N NaOH in ml 5 5 5 5 5 Karmen Units 27 61 114 190 0 SGOT substrate in ml • 4 Blank Protocol for SGOT determination: Test Blank SGOT substrate in ml 0.5 0.5 Incubate at 370C For 5 min Serum in ml 0.1 - Incubate at 37 C For 60min DNPH in ml 0.5 o.5 0 Serum in ml 0.1 Mix and keep At Room Temp. For 20 minutes 0.4 N NaOH 5 5 Mix and keep at room temperature for 10 minutes. Read the intensity of the test against blank at 540 nm. Clinical Pathology and Biochemistry : 58 • Calculations: Plot the graph of O.D. on Y axis and Karmen units on X axis. It is a standard curve. Take the O.D. of serum sample and make a perpendicular line on X axis to find out the enzyme activity of a given sample. • • • Questions for practice What is the normal range of SGOT and SGPT Write the clinical significance of SGOT & SGPT Clinical Pathology and Biochemistry : 59 Chapter 2 : Phosphatase • • • Learning objectives. To study the clinical significances of acid phosphates. To understand the importance of alkaline phosphatase during development. • Introduction: This is a group of enzymes that removes a phosphate group from its substrate. These enzymes hydrolyse the phosphoric acid monoesters into a phosphate ion and a molecule with a free hydroxyl radical. The process of removing the phosphate group is called Phosphatases. The clinically important phosphatases are a group of enzymes which are characterized by their ability to hydrolyse different organic phosphate esters such as para-nitro-phenol phosphate, phenyl phosphate, sodium beta glycerol-phosphate etc. clinically three types of phosphatases are recognized, 1) Alkaline phosphatases with optimum pH 9.8 2) Acid Phosphatase with optimum pH 4.9 3) Red cell phosphate with optimum pH 5.5 to 6. Alkaline phosphatase also called as phosphates working in alkaline medium.(ALP, ALKP) is a hydrolase enzyme responsible for removing phosphate groups from many types of molecules, including nucleotides, proteins, and alkaloids. It is present in bone, liver. Acid phosphatase present mainly in prostate. • (A) Determination of acid phosphatase: • Clinical significance: The Acid phosphatase enzyme shows its action in an acidic PH. It is present in prostate. In the prostatic cancer the values of acid phosphatase increases. Slight to moderate increase is also observed in Paget’s disease and hyperparathyroidism. • • Method: Para-nitro-phenol phosphate Principle: This enzyme acts on para-nitro-phenyl phosphate in citrate buffer at pH 4.9 and liberate nitro-phenol. The liberated nitro-phenol reacts with NaOH and the activity of enzyme is measure. This is the determination of total acid phosphatase. To determine prostatic acid phosphatase the assay is carried out in the presence of tartarate reagent where prostatic fraction gets inhibited. The difference between total and non-prostatic fraction give the reading of prostatic fraction. Clinical Pathology and Biochemistry : 60 • Normal range: Total acid Phosphatase: 0.9 to12 I.U Prostatic acid phosphatase: 0 to 4 IU • Specimen: Clear serum • Reagents: Citrate buffer of pH 4.9 Substrate solution (PNP): 0.4 gram of P- nitro- phenyl phosphate in 100 ml distilled water. 0.1 N NaOH Tartarate solution: 1.5 gram of tartaric acid in 250 ml citrate buffer. Nitro-phenol standard: 4.173 mg/dl Addition table: • Preparation of standard graph: 1 2 3 4 5 Blank Working substrate in ml 0.95 0.9 0.85 0.8 0.7 1.0 Nitro-phenol std. in ml 0.05 0.1 0.15 0.2 0.3 0 Distilled water 0.2 0.2 0.2 0.2 0.2 0.2 0.1 N NaOH 4 4 4 4 4 4 ACP IU 2.5 5 7.5 10 15 0 Keep at room temperature for 5 minutes and read absorbance at 405 nm. Prepare the graph by plotting I.U on X axis and O.D on Y axis • Addition table for serum sample: Working substrate in ml Test total N.P test Blank 1 1 1 Tartarate solution inml 0.2 o Keep at 37 C for 5 min. Serum in ml 0.2 0.2 - Serum in ml - - 0.2 0.1 N NaOH in ml 4.2 4.0 4.2 o Mix and keep at 37 C for 30 min Clinical Pathology and Biochemistry : 61 Mix and incubate for 5 min. at room temperature. Read the absorbance at 405nm and extrapolate on the std. graph to obtain the result. • • (B)Determination of Alkaline Phosphatase: Clinical significance: It is useful in the diagnosis of two groups of conditions 1) Hepato-biliary disease 2) Bone disease associated with increase osteoblast activity In hepatic conditions liver disorders the values are increases but more mark increase is seen in post hepatic jaundice. In Paget’s disease serum ALP values are increases but only smaller increase is found in osteomalacia. In Rickets the values of ALP increases four to five times than normal values. Very high values are found in bone cancer. In hyperparathyroidism moderate elevations of ALP are observed. • Method: P- Nitro-phenol phosphate. Normal range of this method: 20 to 90 I.U. (adults) and 93 to 221 I.U (Children) • Principle: Para-nitro-phenol phosphate is a substrate for the alkaline phosphatase enzyme. It is colourless Alkaline phosphate cleaves Para-nitro-phenol phosphate this into the phosphate and p-nitro-phenol. Under alkaline condition the liberated p-nitro-phenol is converted to p-nitro-phenoxide ions and yellow colour appears. The intensity of yellow colour is directly proportional to the presence of ALP in the serum sample. • Reagents: AMP buffer: pH is 10.3. 78.5 ml of 2 amino -2 methyl -1 propanol in 18 ml concentrated HCl and final volume 1 litre with distilled water. Magnesium chloride 30mg/dl, 0.25 N NaOH Para-nitro-phenol phosphate: Prepare fresh 42.5 mg PNP in0.5 ml of magnesium chloride. P-nitro-phenol standard Working substrate: Procedure: wavelength: 405 nm, and incubation time 15 minutes at Temp. 370C Addition table: • Test Blank AMP buffer in ml 2.7 PNP in ml 0.2 Serum in ml 0.1 2.7 Mix properly. Read initial absorbance and at the same time start stop watch and read the absorbance exactly after 1min, 2 min and 3 min. calculate the mean absorbance Clinical Pathology and Biochemistry : 62 • Calculations: 1595 × Mean absorbance The following conditions or diseases may lead to reduced levels of alkaline phosphatase: • • • • • • • • Hypophosphatemia, an autosomal recessive disease Postmenopausal women receiving oestrogen because of osteoporosis Men with recent heart surgery, malnutrition, magnesium deficiency, hypothyroidism, or severe anaemia. Children after a severe episode of enteritis. Pernicious anaemia. Aplastic anaemia. Chronic myelogenous leukaemia. Wilson's disease. • • • • Questions for practice Write the normal range of Acid phosphatase and alkaline phosphatase. What is the clinical significance of acid phosphatase? What is the principle of the determination of alkaline phosphatase? Clinical Pathology and Biochemistry : 63 Chapter 3 : Dehydrogenase • • • Learning objective: To know about the iso-enzymes of LDH. To understand the important of LDH. • Introduction: A dehydrogenase is an enzyme that oxidizes a substrate by a reduction reaction that transfers one or more hydrides (H−) to an electron acceptor. Lactate dehydrogenase is of significance because it is found extensively in body tissues, such as blood cells and heart muscle. Because it is released during tissue damage, it is a marker of common injuries and disease. • Function: A dehydrogenase is an enzyme that transfers hydrogen from one molecule to another and enzyme Lactate dehydrogenase catalyzes the conversion of pyruvate to lactate and back, as it converts NADH to NAD+ and back. • Iso-enzymes of lactate de-hydrogenases: It exists in four distinct enzyme classes. Functional lactate dehydrogenase is homo or hetero tetramer composed of M and H protein subunit: LDH-1 (4H)—in the heart and in RBC (red blood cells) • • • • LDH-2 (3H1M)—in the reticulo-endothelial system LDH-3 (2H2M)—in the lungs LDH-4 (1H3M)—in the kidneys, placenta, and pancreas LDH-5 (4M)—in the liver and striated muscle[2] Clinical Pathology and Biochemistry : 64 The major isoenzymes of skeletal muscle and liver, M4, has four muscle (M) subunits, while H4 is the main isoenzymes for heart muscle in most species, containing four heart (H) subunits. The other variants contain both types of subunits. Usually LDH-2 is the predominant form in the serum. A LDH-1 level higher than the LDH-2 level suggests myocardial infarction (damage to heart tissues releases heart LDH, which is rich in LDH-1, into the bloodstream • Importance of LDH: It is a protein that normally appears throughout the body in small amounts. Many cancers can raise LDH levels, so LDH may be used as a tumour marker, but at the same time, it is not useful in identifying a specific kind of cancer. Measuring LDH levels can be helpful in monitoring treatment for cancer. Noncancerous conditions that can raise LDH levels include heart failure, hypothyroidism, anaemia, and lung or liver disease. Tissue breakdown releases LDH, and therefore LDH can be measured as a surrogate for tissue breakdown, e.g. haemolysis. Other disorders indicated by elevated LDH include cancer, meningitis, encephalitis, acute pancreatitis, and HIV. LDH is measured by the lactate dehydrogenase (LDH) test). Comparison of the measured LDH values with the normal range helps to guide the diagnosis. • Medicinal Importance of LDH: In medicine, LDH is often used as a marker of tissue breakdown as LDH is abundant in red blood cells and can function as a marker for haemolysis. A blood sample that has been handled incorrectly can show false-positively high levels of LDH due to erythrocyte damage. It can also be used as a marker of myocardial infarction. Following a myocardial infarction, levels of LDH peak at 3–4 days and remain elevated for up to 10 days. In this way, elevated levels of LDH (where the level of LDH1 is higher than that of LDH2) can be useful for determining whether a patient has had a myocardial infarction. It is use in the assessment of tissue breakdown. Useful to follow up lymphoma cancer patients, as cancer cells have a high rate of turnover with destroyed cells leading to an elevated LDH activity. High levels of lactate dehydrogenase in cerebrospinal fluid are often associated with bacterial meningitis. In the case of viral meningitis, high LDH, in general, indicates the presence of encephalitis and poor prognosis. • Determination of LDH: • Clinical significance: LDH is present in the liver and cardiac muscles. Increase values of LDH with SGOT values give an indication of the liver diseases. But LDH play a major role in the differential diagnosis and monitoring of myocardial infarction. The values of LDH beginning within 6 to 12 hours of the infarct and reaching maximum at 48 hours and reaches to normal value after 12 hours. In infective hepatitis the LDH values are increases. The increase is also found in leukaemia, pernicious anaemia. • Method: UV kinetic Normal values by this method: 70 to 240 I.U. Enzyme unit: The enzyme units are expressed as micromoles of pyruvate formed per minute per litre of the specimen. Clinical Pathology and Biochemistry : 65 • Principle: LDH catalyzes the reaction in which lactate in the presence of NAD get converted to Pyruvate and NADH. Increased O.D is measured after 45 seconds by the interval of 1 minute. • • Specimen: Clear serum Reagents: Buffer substrate: 125 ml of glycine buffer, 75 ml of 0.1 N sodium hydroxide and 4 grams of lithium acetate. Mix it and adjust the pH to 10.0. NAD solution: 10 mg dissolve in 0.2 M nicotinamide solution. • Procedure: Wavelength: 340 nm, Cuvette 1 cm light path, Temperature 37oC. Pipette into cuvette as follows. Buffered substrate 1.0 ml NAD solution 0.2ml Serum 0.02 ml Mix and take reading after 45 seconds and then by the interval of 1,2and 3 minutes. And determine the mean absorbance change per minute • Calculations: LDH IU = 9807×mean absorbance change Questions for practice • • • • Write about the iso-enzyme of LDH. What is the importance of LDH Write the clinical significance of LDH What is the principle behind the determination of LDH? Clinical Pathology and Biochemistry : 66 Chapter 4 : Creatinine kinase (CK) • Introduction: It is also known as creatine phosphokinase (CPK) or phosphocreatine kinase, expressed by various tissues and cell types. • Function: CKcatalyses the conversion of creatine to phosphocreatine with the help of ATP and adenosine diphosphate (ADP) is formed.This CK enzyme reaction is reversible and thus ATP can be generated. • Importance of creatine kinase: In tissues and cells that consume ATP rapidly, especially skeletal muscle, but also brain, photoreceptor cells of the retina, hair cells of the inner ear, spermatozoa and smooth muscle, phosphor-creatinine serves as an energy reservoir and source , regeneration of ATP. Thus creatine kinase is an important enzyme in such tissues. Clinically, creatine kinase is assayed in blood tests as a marker of myocardial infarction (heart attack), severe muscle breakdown, muscular dystrophy and in acute renal failure. • Iso-enzymes of creatine kinase: In the cells, the "cytosolic" CK enzymes consist of two subunits, which can be either B (brain type) or M (muscle type).There are three different iso-enzymes: CK-MM, CK-BB and CK-MB. Iso-enzyme patterns differ in tissues. CK-BB is expressed in all tissues at low levels and has little clinical relevance. Skeletal muscle expresses CK-MM (98%) and low levels of CK-MB (1%). The myocardium (heart muscle), in contrast, expresses CK-MM at 70% and CK-MB at 25–30% • Functions: The mitochondrial creatine kinase (CKm) is present in the mitochondrial inter-membranes space, where it produces phospho-creatine from mitochondrial generated ATP and creatine (Cr) imported from the cytosol. There are three cytosolic CK iso-forms present in the cytosol, depending on the tissue. Whereas MM-CK is expressed in skeletal muscle and cardiac muscle, MB-CK is expressed in cardiac muscle, and BB-CK is expressed in smooth muscle and in most non-muscle tissues. Clinical Pathology and Biochemistry : 67 • Medicinal important of CK: CK is often determined routinely in a medical laboratory. It is also determined specifically in patients with chest pain or if acute renal failure is suspected. Normal values are usually between 60 and 174 IU/L, where one unit is enzyme activity, more specifically the amount of enzyme that will catalyse 1 micromole of substrate per minute under specified conditions (temperature, pH, substrate concentrations and activators. Elevation of CK is an indication of damage to muscle. It is therefore indicative of injury, myocardial infarction, myositis and myocarditis. The use of statin medications, which are commonly used to decrease serum cholesterol levels, may be associated with elevation of the CPK level. There is an inverse relationship in the serum levels of T3 and CK in thyroid disease. In hypothyroid patients, with decrease in serum T3 there is a significant increase in CK. Therefore, the estimation of serum CK is considered valuable in screening for hypothyroid patients. Lowered CK can be an indication of alcoholic liver disease and rheumatoid arthritis. Iso-enzyme determination has been used extensively as an indication for myocardial damage. CK can be used in the diagnosis of neuroleptic malignant syndrome. • Determination of Creatine kinase (CK or CPK) • Clinical significance: CPK is activity is highest in brain, heart muscle and skeletal muscle. Increased activity is important in the diagnosis of muscular dystrophy and myocardial infarction. In poly-myositis, motor neuron disorder in acute cerebro-vascular accident the value of CK increases. • • Method: Modified Huges Colorimetric method. Normal values: Men: 20 to 50 IU Women: 10 to 37 IU • Principle: Creatine phosphokinase catalyses the formation of creatine from creatine phosphate.Thecreatine formed in this reaction is react with diacetyl and alpha naphtol in alkaline medium to give coloured complex. The intensity of which is directly proportional to the CPK in the blood sample. • • Specimen: serum. Reagents: Creatine phosphate: 3 mg of creatine phosphate in 1 ml of distilled water add 0.4 ml of Tris buffer and 2 drops of 0.1 N NaOH mix well. Add 24 mg of cysteine hydrochloride and mix properly. ADP: 0.75 mg ADP in 0.3 ml Distilled water. Tris Buffer: 12.1 g tris in 820 ml o0.1 N HCl and make 1 litre using distilled water. 4.9 N Sodium Hydroxide. P-Chloromercuri benzoic acid: 1.07 g/dl in 25 ml of 1 N NaOH add 22 ml of 1N HCl and 53 ml of D.W. Alkaline EDTA: 1.0 gram of EDTA in 10 ml of 1N NaOH and make final volume 1 litre using D.W Clinical Pathology and Biochemistry : 68 Sodium hydroxide: 3N Sodium carbonate: 6N Alpha naphthol: 50 mg of it is dissolve in 1.5 ml of 3N NaOH and 1.5 ml ^N Sodium carbonate. Diacetyl stock: 1 gram /dl and dilute 1:20 before use. Standard: 29.8 mg of creatine monohydrate in 100 ml of alkaline EDTA. • Addition table Test standard Blank Substrate in ml 0.4 0.4 0.4 Distilled water in ml 0 0.2 0.2 Serum in ml 0.05 - 0.05 ADP in ml 0.2 - - Mix well and incubate At 37 oC For 30 min Standard in ml - 0.05 - P chloromercuric benzoic acid 0.5 0.5 0.5 Alkaline EDTA in ml 3.75 3.75 3.75 Alpha Napthol in ml 1.0 1.0 1.0 Diacetyl reagent in ml 0.5 0.5 0.5 Mix well and keep in dark at room temperature for 30 minutes, measure the O.D at 520 nm. • Calculation: CPK activity IU= (O.D of Test – O.D. of blank) ÷ (O.D of std.- Blank) × 66.7 • • Questions for practice Write the normal range and clinical significance of CPK. Clinical Pathology and Biochemistry : 69 • Creatine kinase –MB • Importance of Creatine Kinase- MB Although CK-MB is a sensitive marker for myocardial injury, skeletal muscle has both higher total CK activity per gram of tissue and may be composed of up to 3% CK-MB. When patients experience an MI, the first rise in CK-MB occurs within 4-6 hours after the onset of symptoms. But for diagnosis with high sensitivity and specificity serial sampling over a period of 8-12 hours is required. It has been shown that CK-MB had a clinical sensitivity of 96.8% and a clinical specificity of 89.6% when assayed from samples obtained 12-48 hours after the onset of symptoms of myocardial infarction on admission. • Determination of Iso-enzyme CK-MB • Clinical Significance Creatine kinase is an enzyme consisting of 3 major iso-enzymes .They are as follows 1) CK-BB(Brain) 2) CK-MB(Heart) 3) CK-MM (Skeletal Muscle). In the myocardial infarction blood levels of both CK- Total & CK-MB usually increase markedly but only CK-MB elevation is highly specific for the diagnosis of MI. CK-MM increases following muscular trauma & exercise. CK-BB is rarely detected in serum. • Method Column Method: • Principle: The CK-iso-enzymes are absorbed on sephadex A-50. By using imidazole buffer (pH: 6.7) CK-MB fraction is eluted & determined by end point reaction or by kinetic method. Questions for practice : • Write the normal range and clinical significance of CPK MB. Clinical Pathology and Biochemistry : 70 Chapter 5 : Electrolytes • • • Learning Objectives To understand the operation of flame photometer To know the normal value of sodium, potassium. • Introduction: Water is an important compound of living cell, powerful solvent for many ionic compounds and neutral molecules; It shows major influence on the structural and functional components of the cell. Total body fluid is distributed in between two compartments i.e. extracellular fluid and intracellular fluid .The fluid outside the cell is extracellular and which is inside the cell is intracellular. Electrolytes are important substances which influences the distribution and retention of water. Sodium the chief cation of the extracellular fluid and potassium is the chief cation of the intracellular fluid. These two compounds are also termed as osmotically effective electrolytes. • Sodium: It is a major component of the cations of the extracellular fluid, largely associated with chloride and bicarbonate and acid base balance. • Source: Sodium chloride use in cooking the food, salted food, cheese, bread, carrot, eggs, milk, nut, radishes etc. • Requirement: About 4 grams of sodium is ingested every day and about 95% is excreted in urine by sweat glands, salivary glands and the gastrointestinal tract. Kidney play important role in the retention of it. • Clinical significances: Decrease sodium level is termed as Hyponatremia. It is due to the following reasons. • Less intake of Sodium. • Loss of water and sodium from the body. • Burns and massive sweating. • Addison’s disease deficiency of mineralocorticoids. • Diabetes ketoacidosis • Prolonged vomiting and diarrhoea. Due to high loss of sodium from the body is concentration in the extracellular fluid decreases and fluid become hypotonic. Water leaves extracellular fluid and more water is lost from the tissue fluid. Symptoms of the above conditions are, Vaso-constrictive shock Nausea, Vomiting, cramps, risk of circulatory failure • Treatment: Saline injection Increase sodium level is termed as Hypernatremia. It is due to the following reasons, • Excess intake of sodium chloride mostly intravenously • In Cushing’s syndrome where there is excess release of mineralocorticoids Clinical Pathology and Biochemistry : 71 Symptoms are, • There is raised central venous pressure • Peripheral oedemas • Pulmonary oedema with eventual respiratory failure. • Potassium : • Introduction. It is the main cation of the intracellular fluid within the cell it play important role in maintenance of acid base balance, osmatic pressure and water retention. It is also useful in catalysing many enzymatic reactions. It is also present in the extracellular fluid and influence the cardiac muscle activity. • Source: The normal intake is 4 grams /day. Main sources are chicken dried apricots, bananas, dried peaches, oranges, pineapples, potatoes etc. Metabolism is controlled by mineralo-corticoids. It is excreted through kidney. • Clinical significances: Elevated levels of serum potassium are termed as hyper-kalaemia is observed in the following conditions. • Renal failure. Highly dehydration Shock Addison’s disease • The symptoms of the elevated levels of serum potassium are, • Depression mentally. Cardiac system depression Mental confusion Weakness of respiratory muscles Numbness and weakness • • • • • • • Low potassium is termed as Hypokalemia a deficiency in the potassium .This condition is due to the following reasons, • Gastrointestinal loses • Malnutrition • Cushing’s syndrome • Metabolic alkalosis • The Symptoms of the low level of potassium are, • Muscle weakness Irritability Paralysis Dilation of the heart with change in ECG. • • • Clinical Pathology and Biochemistry : 72 • Determination of electrolytes: Flame photometer: • Clinical significance: Hyponatremia is the low serum sodium value. This condition is observed in prolonged diarrhoea and vomiting etc. Hypokalaemia means low serum potassium values observed in Cushing’s syndrome, renal tubular damage, metabolic alkalosis and malnutrition. Hypernatremia means increase in the serum sodium values severe dehydration, loss of dilute urine in diabetes insipidious, salt poisoning, Cushing’s syndrome. Hyperkalaemia means high serum potassium values which are observed in a condition such as renal glomerular disease, Addison’s disease, and oliguria. • Normal values: Serum Sodium: 133 to148 m Eq /l Serum Potassium: 3.8 to5.6 m Eq /l • Specimen: Clear serum • Requirement: distilled water, Sodium / potassium standards: 120/2.0 m Eq/l, 140/4.0 m Eq/l, 160/6 m Eq/l • Principle: The test solution is passing under flame photometer as a fine non luminous flame where solution evaporates and ions are formed. These ions emits light of characteristic wavelength .The flame is simultaneously monitored by both channels. Each channel consist of photo detector outputs of which are connected to the digital display, Clinical Pathology and Biochemistry : 73 • Procedure: Pipette in a tube labelled as follows, Test Std. 1 Std. 2 Glass distilled water 10 10 10 Serum or plasma 0.1 Std. 120/2 0.1 Std. 140/4 0.1 Std. 160/6 Std. 3 10 0.1 Mix and transfer to the beakers for the flame photometer determination. • Operation of the flame photometer: Put main switch on and air compressor, adjust the required air pressure and introduce glass distilled water through atomizer. Put on gas and control the flame fine luminous blue. Adjust the proper filter for sodium and potassium determination and make zero adjustment introducing glass distilled water. Introduce 120/2 standard and set the standard, repeat the procedure for remaining standard also to set the reading. Introduce the test sample and take the reading. Note the result. Questions for practice • Write the principle of the electrolyte determination, • What are the normal values of sodium and potassium? • What are the clinical significances of serum sodium and potassium? Clinical Pathology and Biochemistry : 74 Chapter 6 : Bicarbonates • • • Learning Objectives To calculate the bicarbonate level in the blood sample. To know about the respiratory acidosis and alkalosis. • Introduction: When the gases of the inspire air come in contact with the alveolar membrane of the lungs the exchange of gases takes place. During the exchange it obeys laws of diffusion. Oxygen tension in alveolar air is 107 mmHg and in venous blood it is 40 mm Hg the difference of 67 mmHg is required to drive oxygen from the alveoli of the lungs into the blood. The CO2 tension in alveolar air is 36 mm Hg and of venous blood it is 46 mm Hg the difference of 10 mmHg is sufficient to drive CO2 from the blood into the lungs. Buffer systems present in the body are responsible to maintain the normal pH of the body even the concentration of CO2 increases this system is bicarbonate buffer system. The CO2 formed in the metabolic reactions enters the blood and dissolves in water to form H2CO3 which dissociate and formed HCO3-- AND H+. HCO3-- get combine with cation such as Na and formed NaHCO3 and H+ is get accepted by reduced haemoglobin. In the lungs when blood returns for purification reduced haemoglobin combines with oxygen and forms oxy-haemoglobin with release of H ions. This hydrogen is also ready to combine with the HCO3--ions to from H2CO3 . Carbonic anhydrase acts on H2CO3 to form CO2 and water where CO2 expelled out in the expired air. Because of the disturbances in the content of H2CO3 in the blood which is due to CO2 gas the following clinical conditions are seen. • Clinical conditions: Respiratory acidosis: Accumulation of H2CO3 in the blood in conditions such as asthmas, Pneumonia, emphysema, depression of the respiratory canter. • Respiratory alkalosis: Decrease in the carbonic acid reaction but no corresponding changes in the bicarbonate ions. This condition is brought about by forced hyperventilation which washes abnormally large quantities of CO2. Clinical observed in high fever, Hepatic coma, Encephalitis, during anaesthesia. • Metabolic acidosis is a condition caused by decrease in the bicarbonate fraction with relatively smaller change in the H fraction. This condition is due to uncontrolled diabetes, renal diseases, Poison by acid, excessive loss of intestinal fluid, ingestion of ammonium chloride. • Metabolic alkalosis: Increase in the bicarbonate fraction with relatively smaller change in the carbonic acid fraction. Due to Citrate alkali ingestion, prolonged vomiting. • Clinical Pathology and Biochemistry : 75 Normal values: • Arterial PCO2 : 35 -45 mm hg ( 1.02 to 1.35 moles/l) • Arterial PO2 : 95- 199 mmHg • Standard bicarbonate : 21 to 28 m Eq/L Carbon dioxide combining power is 53 to 75 ml per 100 ml of plasma. • 1) Determination of blood pH, PCO2 PO2 and bicarbonates: • Blood gas analyser: • Introduction: The arterial blood is used to determine blood gas. By this pH, PCO2 standard bicarbonate and excess or deficiency of base can be determined. A single blood sample is in contact with, PCO2 and PO2 electrodes simultaneously. Important component of blood gas analyser are the PCO2 electrode, PO2 electrode. PCO2 electrode consists of pH sensitive glass electrode and a reference silver – silver electrode immersed in a bicarbonate buffer system. It is covered with the membrane permeable to only CO2 gaseous present in the blood sample. After application of blood sample co2 present in the sample diffuses from this membrane and reacts with the bicarbonate buffer system. The pH of system gets change. This dissolution of CO2 is detected by the pH sensitive glass electrode .a potential difference exists between the glass electrode and the reference electrode and measure on the meter. The meter’s scale is usually calibrated for PCO2 in semilogarithmic fashion since pH is inversely proportional to the log of the PCO2. PO2 electrode: A platinum and silver- silver chloride electrode is immersed in a phosphate and sodium chloride buffer. These electrodes are covered with a membrane which permits only gaseous oxygen no other ions. After diffusion an electro-reduction reaction takes place and the measure. Measurements: pH of the blood: Heparinised whole arterial blood is used and determine immediately after collection of blood sample. It is Clinical use to assign uncompensated acidosis or alkalosis. • TCO2S, Total CO2: Measurement of CO2, carbonic acid, and bicarbonate of plasma. Blood plasma collected under liquid paraffin gives measure of CO2 content and reported as CO2 per 100 ml at standard conditions of temperature and pressure. • pCO2: The plasma carbonic acid is determining by measuring the PCO2 .The PCO2 of the arterial blood is directly proportional to the amount of CO2 produced in the body. • PO2: To assess the oxygen carrying capacity of blood haemoglobin. If elevated PO2 levels values are seen it indicates the decreased oxygen affinity of the haemoglobin. Low values are measure of anoxia. • • Clinical Pathology and Biochemistry : 76 • The plasma bicarbonates: • Introduction: It is influenced by the change in PCO2 and the degree of oxygen saturation and is alkali reserve. It is utilized to neutralize all the acidic compounds entering in the blood and tissue. • Determination of the plasma bicarbonates • Normal range: 21 to 28 mEq/l • Method: It is determined by titrimetric method using 0.01N hydrochloric acid. • Principle: Serum is added to standard 0.01N hydrochloric acid and the loss of strength of the standard acid due to bicarbonate is determined by titrating against 0.01N NaOH. • Specimen: 3 ml o blood is collected without anticoagulant and serum is separated. • Procedure: 1) 5 ml of 1 gram /dl saline, add 0.1 ml of specimen and drop of phenolphthalein indicator mix titrate using 0.01N NaOH till colour develop. 2) 4 ml of 1 gram/dl saline, 1 ml of o.o1N HCl 0.1 l of serum and titrate using 0.01N NaOH, the colour changes from yellow to red same as in the case of control. Note the reading as R • Calculation: 1-R ×100 Questions for practice : • Write significance of blood gases &bicarbonate Clinical Pathology and Biochemistry : 77 Unit 4 : Biochemical Test Profile Learning Objective • LFT: To learn the different functions of Liver. • You can calculate the severity of Jaundice. • Assessment of Liver condition in different disease. • RFT • Endocrine function test • Lipid profile • Amylase • GTT Clinical Pathology and Biochemistry : 78 Chapter 1 : Liver Function Tests The liver is the largest organ in the human body weight 1.2 to 1.5 kg in adult. • Liver Functions: • • • • • • • Excretory function: Liver plays an important role in the bile formation and excretion of bile into the intestine, secretion of products in the bile for e.g. bile salts, bilirubin conjugates and cholesterol. Excretion of the substances by hepatic activity e.g. heavy metals dyes. Metabolic functions: Liver is a center for the metabolic activity. Metabolism of carbohydrate, protein and lipids take place in the liver. Excess glucose get converted in to glycogen. Lipids and proteins metabolic products are formed in the liver. Liver is a site for gluconeogenesis. Protective function: Kuffer’s cells remove foreign bodies from blood. Detoxification: Detoxification of toxic substances by conjugation, methylation oxidation reduction. Removal of ammonia by converting into urea and excreted. Hematologic function: Liver is site for protein synthesis. In the blood coagulation process different factors are requires like fibrinogen, prothrombin, heparin. These are synthesized in liver. Destruction of erythrocytes takes place in liver. Circulatory function: transfer of blood from portal to systemic circulation, regulation of blood volume. Bile pigment metabolism: Synthesis and secretion of conjugated bilirubin takes place in the liver. • Liver function tests (LFTs or LFs) : • Introduction: These are groups of clinical biochemistry laboratory blood assays designed to give information about the condition of a patient's liver. The parameters measured include Prothrombin time (PT/INR), aPTT, albumin, bilirubin (direct and indirect) and others. Liver transaminases (AST (SGOT) and ALT (SGPT)) are useful biomarkers of liver injury in a patient with some degree of intact liver function. Most liver diseases cause only mild symptoms initially, but it is important that these diseases be detected early. Hepatic (liver) involvement in some diseases can be of crucial importance. This testing is performed by a medical technologist on a patient's serum or plasma sample obtained by phlebotomy. Some tests are associated with functionality (e.g., albumin); some with cellular integrity (e.g., transaminase) and some with conditions linked to the biliary tract (gamma-glutamyl transferase and alkaline phosphatase). Several biochemical tests are useful in the evaluation and management of patients with hepatic dysfunction. These tests can be used to (1) detect the presence of liver disease, (2) distinguish among different types of liver disorders, (3) gauge the extent of known liver damage, and (4) follow the response to treatment. Some or all of these measurements are also carried out (usually about twice a year for routine cases) on those individuals taking certain medications anticonvulsants are a notable example in order to ensure that the medications are not damaging the person's liver. Clinical Pathology and Biochemistry : 79 • Different liver test based on the different functions of the liver. (A) Tests based on abnormalities of pigment metabolism: 1) Serum bilirubin : Total , Direct , Indirect Bilirubin 2) Urine bilirubin (B) Liver Enzymes 1) SGPT 2) SGOT 3) ALP 4) GGT (C) Tests Based on Changes in Plasma Proteins 1) Estimation of total plasma proteins, albumin and globulin and determination of Albumin: Globulin ratio. (D) Tests Based on Abnormalities of Lipids 1)Cholesterol-Cholesterol esters Ratio (E) Formation of Prothrombin by Liver (F) Tests based on amino acids catabolism 2) Determination of blood NH3 • Albumin: Albumin is a protein made specifically by the liver. It is the main constituent of total protein (the remaining from globulins). Albumin levels are decreased in chronic liver disease, such as cirrhosis. It is also decreased in nephritic syndrome, where it is lost through the urine. • Transaminases: Aspartate transaminase (AST) also called serum glutamic oxaloacetate transaminase (SGOT) or aspartate aminotransferase (ASAT) is similar to ALT in that it is another enzyme associated with liver parenchymal cells. It is raised in acute liver damage, but is also present in red blood cells and cardiac and skeletal muscle and is therefore not specific to the liver. Elevated AST levels are not specific for liver damage, and AST has also been used as a cardiac marker. • Alkaline phosphatase: Alkaline phosphatase (ALP) is an enzyme in the cells lining the biliary ducts of the liver. ALP levels in plasma will rise with large bile duct obstruction, intrahepatic cholestasis or infiltrative diseases of the liver. ALP is also present in bone and placental tissue, so it is higher in growing children and elderly patients with Paget's disease. • Bile pigment metabolism and Total bilirubin: Erythrocytes at the end of their life span are destroyed in the reticulo-endothelial system, globulin is separated from it iron is released stored, and porphyrin ring of hemoglobin is open. Green colour biliverdin is formed from porphyrin ring i.e. from non-iron part of hemoglobin. Biliverdin get reduced to yellow colour bilirubin is water insoluble and it is called indirect bilirubin. It circulates in the blood with albumin. In the liver albumin separated from it and biliverdin gets conjugated with glucuronic acid to form water soluble direct bilirubin. It is excreted in the urine. This reaction is Clinical Pathology and Biochemistry : 80 catalyzed by bilirubin UDP glucuronic transferase. Conjugated bilirubin is excreted into the biliary canaliculi and then through bile duct it passes to the intestine. Fig 4.1.1 Bilary system In the large intestine it is reduced by bacterial action to urobilinogen, excreted into the urine. The liver is responsible for clearing the blood of un-conjugated bilirubin. The yellow colour for urine is because of metabolism of bilirubin into urobilinogen followed by its re-absorption. Further metabolism of urobilinogen into stercobilin while in the large intestine accounts for the brown colour of stool. Increased total bilirubin (TBIL) causes jaundice, and can indicate a number of problems: • 1) Pre-hepatic: Also called haemolytic jaundice, occur due to the excessive destruction of red blood cells which increases bilirubin production. This is due to haemolytic anaemia and internal haemorrhage. It may be due to the increased formation of indirect bilirubin than the normal liver can convert it into direct bilirubin there is no hepatic damage. Its causes are Sickle cell disease, Thalassemia of red blood cells, Glucose 6 phosphate deficiency and outside the RBC’s due to haemolytic jaundice, leukaemia, and infections like malaria and physical agents like burns. • Clinical features of pre-hepatic jaundice: Lemon yellow tinge to the skin-mucosa. Anaemia, decrease hemoglobin, chronic ulcers and excess activity of reticulo-endothelial system. • General laboratory observations in pre-hepatic jaundice are: Serum total bilirubin increases direct bilirubin normal and indirect bilirubin increases. In the urine examination bile salts and bile pigments are absent but uro-bilinogen is high. In the stool examination there is dark colour of the stool. Clinical Pathology and Biochemistry : 81 • 2) Hepatic: It is also called hepato-cellular jaundice. Problem is with the liver, which is reflected as disorder of the liver cells and bile passage within liver. There is ineffective transport of bilirubin within cells, defective conjugation and retention of indirect bilirubin. It also includes jaundice caused by infection of viruses called infective hepatitis. Viruses that cause hepatitis include hepatitis A virus, Hepatitis B virus, hepatitis C virus, hepatitis D virus, and hepatitis E virus Epstein-Barr, herpes simplex virus etc. These viruses are entering in the body through contamination of water, insects like flies, bedbugs. Vectors food contamination, use of infected needles, transfusion etc. are another routes for infective hepatitis. • Clinical features of hepatic jaundice: There are deficiencies in bilirubin metabolism. General functions of the hepatocytes are disturbed. There may be reduced hepatocyte uptake, impaired conjugation of bilirubin, and reduced hepatocyte secretion of bilirubin. Some examples would be cirrhosis and viral hepatitis. Clinical feature is increase in the total bilirubin. • General laboratory observations in hepatic jaundice are: Increase in the total bilirubin concentration with the simultaneous increase in the direct and indirect bilirubin. In the urine sample bile salts bile pigments are present with small increase in the uro-bilinogen. • 3) Post-hepatic: Liver function in this type of jaundice is found to be normal. Hepatocytes function is normal but there is interference in the normal passage of bile to the duodenum. It is due to the obstruction of the bile ducts due to various reasons. • Clinical features of Post- hepatic jaundice: There may be the gall stone in the common bile duct, tumour of the bile duct, atresia of the main bile duct reflected as deficiencies in bilirubin excretion. • General laboratory observations in Post -hepatic jaundice are: The clinical findings in the post hepatic jaundice are increase in the both direct and indirect bilirubin in the serum. There is dark yellow colour to urine and skin. There is deficiency of fat soluble vitamins like A,D, E and K because of poor absorption of fat in the absence of bile salts. • Direct bilirubin (conjugated bilirubin) The diagnosis is possible by looking at the levels of direct bilirubin. If direct (i.e. conjugated) bilirubin is normal, then the problem is an excess of unconjugated bilirubin (indirect bilirubin), and the location of the problem is upstream of bilirubin conjugation in the liver. Hemolysis, viral hepatitis, or cirrhosis can be suspected. If direct bilirubin is elevated, then the liver is conjugating bilirubin normally, but is not able to excrete it. Bile duct obstruction by gallstones or cancer should be suspected. • Gamma glutamyl transferase: Specific to the liver sensitive marker for cholestasis damage than ALP, Gamma glutamyl transpeptidase (GGT) may be elevated with even minor, sub-clinical levels of liver dysfunction. It can also Clinical Pathology and Biochemistry : 82 be helpful in identifying the cause of an isolated elevation in ALP; GGT is raised in chronic alcohol toxicity. • The uses of liver function tests: 1) To diagnose different types of jaundice. 2) To assess the severity of jaundice. 3) To follow the trend of disease. 4) To gauge post-operative risk. 5) To screen Infective hepatitis cases. There are two groups of tests used in clinical laboratory practices. • • Group I tests: Total bilirubin, direct bilirubin and indirect bilirubin, urine analysis for bile pigments, bile salts and urobilinogen. These tests are helpful in differentiation of pre- hepatic jaundice from other types of jaundice. Group 2 tests: SGPT, SGOT, Alkaline phosphatase and gamma -glutamyl transferase. In pre hepatic jaundice in the absence of cellular damage of the liver cells serum SGPT, SGOT and ALP levels are normal. The increase of the SGPT and SGOT is related to severity of liver cell damage and liver cell necrosis. In viral hepatitis very high levels are seen. Mostly there is increase of 10 to 20 times the upper limit of normal range is observed. But when biliary obstruction is present steady elevation of these enzymes is observed than hepatic cells or liver disorder because these enzymes are present in the liver cells. Serum alkaline phosphate (ALP) is excreted through biliary system in the same manner as the bilirubin is excreted via the bile duct. In the pre-hepatic jaundice hepato-biliary system is not affected so its level is normal. In hepatic jaundice due to liver cell disturbance ALP increases. In the toxic hepatitis ALP values are increases. In post hepatic jaundice ten times more than normal values of ALP are observed. The rise of ALP is parallel to the intensity of post hepatic jaundice. • Determination of serum glutamate pyruvate aminotransferase (SGPT/ALT) and Serum glutamate oxaloacetate aminotransferase (SGOT/AST): • Clinical significance: ALT is found predominantly in the liver, with clinically negligible quantities found in the kidneys, heart, and skeletal muscle, while AST is found in the liver, heart (cardiac muscle), skeletal muscle, kidneys, brain, and red blood cells. As a result, ALT is a more specific indicator of liver inflammation, liver damage, liver cirrhosis the disease conditions associated with the liver. AST may also be elevated in diseases affecting other organs, such as myocardial infarction, acute pancreatitis, acute hemolytic anaemia, severe burns, acute renal disease, musculoskeletal diseases, and trauma. AST was defined as a biochemical marker for the diagnosis of acute myocardial infarction. Clinical Pathology and Biochemistry : 83 • Method: End point Reitman and the Frankel’s method. • Enzyme unit: Karmen units: These are expressed as the units of the activity which produces change in the O. D. of 0.001per minute by enzyme present in the 1 ml of the serum. • Principle: The following reaction is catalysed by the enzyme glutamate oxaloacetate transaminases (SGOT).This enzyme catalysed the reversible transfer of amino group from glutamate to oxaloacetate simultaneously replacing the amino group of glutamate to carbonyl group and gets converted to aspartate. In the Reitman-Frankel reaction SGOT catalyzes the reaction of aspartic acid and alpha-ketoglutaric acid to oxalo-acetic acid and glutamic acid. The oxalo-acetic acid is treated with 2,4-dinitrophenylhydrazine in an alkaline medium to produce a highly colored hydrazine which can be measured colorimetric -ally. The following reaction is catalysed by the enzyme glutamate aminotransferase( SGPT).This enzyme catalysed the reversible transfer of amino group from glutamate to pyruvate simultaneously replacing the amino group of glutamate to carbonyl group and get converted to alanine. . The pyruvic acid is treated with 2,4-dinitrophenylhydrazine in an alkaline medium to form a highly colored hydrazine which is measured photometrically. Clinical Pathology and Biochemistry : 84 Preparation of reagents: SGPT substrate: It contains 1.78 gram of alanine, 30 mg of alpha ketoglutaric acid 0.5 ml of 1 N NaOH in a phosphate buffer of pH 7.45.Make the final volume 100 ml using buffer. • SGOT substrate: IT contains 2.66 gram of Aspartic acid, 30 mg of alpha ketoglutaric acid 20 ml of 1 N NaOH in a phosphate buffer of pH 7.45.Make the final volume 100 ml using buffer. • DNPH reagent: 200 mg of Di-nitro-phenyl hydrazine in 0.01N hydrochloric acid 0.4 N Sodium Hydroxide., 22 mg/dl Sodium pyruvate Stability of the reagent: The reagent is stable at 2oC to 80C. • Sample: Un-haemolysed serum sample • Procedure: Preparation of calibration graph for SGPT: • • SGPT substrate in ml Std.Sodium Pyruvate in ml Distilled water in ml DNPH in ml Mix and keep for 20 min at 0.4 N NaOH in ml Karmen Units 1 0.45 0.05 2 0.4 0.1 3 4 0.35 0.3 0.15 0.2 Blank 0.5 - 0.1 0.1 0.1 0.5 0.5 0.5 room Temp. 0.1 0.5 5 28 5 5 150 0 5 57 5 97 0.1 0.5 Protocol for SGPT determination: Test Blank SGPT Substrate in 0.5 0.5 ml Incubate at 370C For 5 min Serum in ml 0.1 Incubate at 370C For 30min DNPH in ml 0.5 o.5 Serum in ml 0.1 Mix and keep At Room For 20 minutes Temp. 0.4 N NaOH 5 5 Mix and keep at room temperature for 10 min. Read the intensity of the test against blank at 540 nm. Clinical Pathology and Biochemistry : 85 • • Preparation of calibration graph for SGOT: 1 SGOT substrate in ml 0.45 Std.Sodium Pyruvate in 0.05 ml Distilled water in ml 0.1 DNPH in ml 0.5 Mix and keep for 20 min room at 0.4 N NaOH in ml 5 Karmen Units 27 2 0.4 0.1 3 4 0.35 0.3 0.15 0.2 Blank 0.5 - 0.1 0.1 0.5 0.5 Temp. 0.1 0.5 5 61 5 5 190 0 5 114 0.1 0.5 Protocol for SGOT determination: Test Substrate 0.5 SGOT in ml Incubate at 370C Serum in ml Incubate at 370C DNPH in ml Serum in ml Mix and keep 0.4 N NaOH For 0.1 For 0.5 At Temp. 5 Blank 0.5 5 min 60min o.5 0.1 Room For 20 minutes 5 Mix and keep at room temperature for 10 min. Read the intensity of the test against blank at 540 nm. • Calculations: Plot the graph of O. D. on Y axis and Karmen units on X axis. And calculate the enzyme activity by extrapolating the absorbance of the serum sample. • Determination of serum bilirubin by Malloy and Evelyn method • Clinical significance: Jaundice may be classified as pre-hepatic, hepatic, or post-hepatic. In pre-hepatic jaundice, excess bilirubin production i.e. haemolysis is responsible. Hepatic jaundice occurs when either the removal of bilirubin from the blood or conjugation of bilirubin by the liver is defective. Hemolytic jaundice also called hepatic is caused by over production of bilirubin due to excessive hemolysis and the inability of the liver to adequately remove this pigment from the blood. This condition is usually associated with elevated values of serum indirect bilirubin. Cirrhosis of the liver and infectious or toxic hepatitis is caused by some type of intrahepatic obstruction, where production of bilirubin is not increased, but accumulates and is discharged back into the blood. In these conditions, the indirect form of bilirubin predominates in the early phase, but as liver damage progresses the direct form also becomes elevated. Clinical Pathology and Biochemistry : 86 Obstructive jaundice, also called as post hepatic caused by a post-hepatic blockage of the larger bile passages, particularly the common bile duct, results in a reflux of bilirubin into the blood. It is associated with elevated serum bilirubin only of the direct type. • Principle: In this procedure of Malloy and Evelyn the Van den Bergh Reaction is applied. In an aqueous solution, diazo reagent reacts with the direct bilirubin in the serum to form a pink to reddish-purple colored compound (azo-bilirubin). It is read at one minute. In a 50% methyl alcohol solution, di-azo reagent reacts with the total bilirubin in the serum to form a pink to reddish-purple colored compound. (Read at 30 minutes.) The difference between total bilirubin and direct bilirubin gives the values of indirect bilirubin. • Specimen: Fresh serum is recommended, but heparinized plasma is also acceptable. • Preparation of the reagent: Diazo A: Prepared by mixing 0.1 gram of Sulphanilic acid in 100 ml of 1.5% HCl Diazo B: 0.5 gram Sodium nitrite in 100 ml Distilled water. Diazo blank reagent: 1.5% Hydrochloric acid. Methanol Bilirubin standard • Addition table: Prepare fresh diazo mix by mixing 5 parts of Diazo A and 0.15 parts of diazo B and use. Total Test Total blank Direct test Direct blank Distilled water in 1.8 ml 1.8 1.8 1.8 Serum in ml 1.8 1.8 1.8 1.8 Diazo mix in ml 0.5 Methanol in ml 2.5 Distilled water 0.5 2.5 2.5 2.5 Keep in dark for 30 min read the intensities at 540 nm. Read the O.D. of artificial standard undiluted bilirubin directly at 540 nm. Clinical Pathology and Biochemistry : 87 • Observations: specimen O.D. at 540 nm Total test Total blank Direct test Direct Blank • Calculations: O.D of total bilirubin= O.D. of total test - O.D. of total blank O.D of direct bilirubin= O.D. of direct test - O.D. of direct blank Total bilirubin mg/dl = (O. D. of total bilirubin ÷ O.D of standard) × 10 Direct bilirubin mg/dl = (O. D. of direct bilirubin ÷ O.D of standard) × 10 Indirect bilirubin = total bilirubin – direct bilirubin • Determination of bilirubin by DMSO method • Reagents: Diazo A also called DMSO = 5 gram sulphanilic acid + 12 ml hydrochloric acid and 512 ml dimethyl sulphoxide in 1 lit water. Sulphanilic acid: 0.5 gram/dl in 1.5% Hydrochloric acid. Diazo B: .5 gram sodium nitrite in 100 ml distilled water. • Addition table for DMSO method of bilirubin determination: Total Test Total blank Direct test Direct blank 2.8 2.8 - - - - 2.8 2.8 Diazo B in ml 0.1 - 0.1 - Serum in ml 0.1 0.1 0.1 0.1 Diazo A in ml Sulphanilic acid in ml Clinical Pathology and Biochemistry : 88 Mix and read the intensities of the tubes against respective blank at 530 nm. • Calculations: O.D of total bilirubin= O.D. of total test - O.D. of total blank O.D of direct bilirubin= O.D. of direct test - O.D. of direct blank Total bilirubin mg/dl = (O.D. of total bilirubin ÷ O.D of standard) × 10 Direct bilirubin mg/dl = (O.D. of direct bilirubin ÷ O.D of standard) × 10 Indirect bilirubin = total bilirubin – direct bilirubin • Normal range: Total bilirubin up-to 1 mg/dl, Direct and indirect bilirubin up-to 0.5 mg/dl • Determination of Total protein and albumin: • Clinical significance: Total protein measurements are used in the diagnosis and treatment of diseases involving the liver, kidney or bone marrow, as well as other metabolic or nutritional disorders. • Method: Endpoint. Biuret method • Principle: In the reaction, the peptide bonds in the protein sample bind to cupric ions in an alkaline medium to form colored peptide/copper complexes. This change in absorbance is directly proportional to the concentration of total protein in the sample. • Specimen: Biological fluid samples should be collected in the same manner routinely used for any laboratory test. Freshly drawn serum or plasma is the preferred specimens. • Normal range: 6to 8 g /dl • Preparation of the reagent: Biuret reagent: 45 gram of Rochelle salt in 400 ml of 0.2N NaOH add 15 gram copper sulphate and5 gram potassium iodide make final volume 1 liter with 0.2N NaOH .Dilute 200 ml of this reagent to 1 lit using 0.2 N NaOH and use. Standard protein: 6 gram/dl Clinical Pathology and Biochemistry : 89 • Addition table: Test Std. Blank Protein reagent in ml 5 5 5 Serum in ml 0.05 - - Standard 6 gram/dl - 0.05 - Distilled water - - 0.05 Mix and incubate at room temperature for 10 minutes. Measure the absorbance at 530 nm. • Calculations: (O.D Of test ÷ O.D of standard) × 6 Serum albumin: Principle: Albumin reacts with Bromo-cresol green at pH4.1 to form green colored complex and the absorbance is measured at 640 nm. This change in absorbance is directly proportional to the concentration of total albumin in the sample. • Normal range: • 3.3 to 4.8 g/dl • Reagents: Add 8.85 gram succinic acid, 108 mg Bromo-cresol green, 100 mg sodium azide, and Brij 35 4 ml in 900 ml distilled water, adjust the pH 4.1 then make final volume 1 litre using distilled water. • • Albumin standard: 4 gram/dl Addition table: Test Std. Blank Albumin reagent in ml 5 5 5 Serum in ml 0.05 - - Standard 6 gram/dl - 0.05 - Distilled water - - 0.05 Clinical Pathology and Biochemistry : 90 Mix and incubate at room temperature for 10 minutes. Measure the absorbance at 640 nm. • Calculations: (O.D Of test ÷O.D of STD) × 4 • Determination of Alkaline phosphatase (ALP) :Visible kinetic method • Clinical significance: Serum ALP levels can greatly increase with liver tumors and lesions and can show moderate increase with hepatitis. • Principle: Alkaline phosphate present in the sample reacts with a substrate para nitro-phenol phosphate (PNPP). This liberates p- nitro- phenol and the phosphate. In visible kinetic method the liberation of PNP is measured. • Normal values by this method: Adults: 20 – 80 IU Children: 93 to 221 IU • Specimen: Plasma free from haemolysis or clear serum • Reagent: AMP buffer: pH is 10.3. 78.5 ml of 2 amino -2 methyl -1 propanol in 18 ml concentrated HCl and final volume 1 lit with distilled water. Magnesium chloride 30mg/dl Para-nitro-phenol phosphate: Prepare fresh 42.5 mg PNP in0.5 ml of magnesium chloride solution. • Procedure: wavelength: 405 nm, Cuvette 1cm light path and Temp. 370C • Addition table: Test AMP buffer in ml 2.7 PNP in ml 0.2 Serum in ml 0.1 Mix properly Read initial absorbance and at the same time start stop watch and read the absorbance exactly after 1min, 2 min and 3 min. calculate the mean absorbance. Clinical Pathology and Biochemistry : 91 • Calculations: The multiplying factor may be slightly different for different kits. 1595 × Mean absorbance / min • Determination of Gamma Glutamyl Transferase.: • Clinical significance: GGT is found in kidney, pancreas, liver and prostate tissues. In Liver damage, liver cirrhosis, hepatitis the GGT level increase. The value of GGT is more helpful than ALP value in liver damage because GGT remains normal in the bone disease. It is more useful than AST because it remain normal in muscle disorders. The values of GGT are more helpful in monitoring the recovery from hepatitis. • Method: End point method • Principle: L.Gamma- glutamyl-p-nitroanilide + glycyl -glycine under the influence of GGT produces para-nitroaniline. • Normal value: For this method: male; 4 to 23 IU Female: 3.5 to 13 IU • Preparation of the reagent: Substrate: 250 mg of L.Gamma- glutamyl-p-nitroanilide, 872 mg of glycyl -glycine and 672 mg of magnesium chloride in 300 ml of AMP buffer of pH 8.6 0.0075 M NaOH. 12.4 mg/dl P-nitro-aniline standard • Preparation of standard graph: 1 2 3 4 5 bl Substrate in ml 0.9 0.7 0.5 0.2 0.1 1 P nitroaniline std. in ml 0.1 0.3 0.5 0.8 0.9 0 Sodium hydroxide 5 5 5 5 5 5 GT in IU 10 30 50 80 90 00 Mix and read the absorbance at 405nm against blank and plot the graph of O.D on Y axis and IU on x axis. Clinical Pathology and Biochemistry : 92 • Addition table of sample; Tests Blank 1 1 0.2 - 5 5 Substrate in ml serum in ml 0 Incubate at 37 C for 45 min Sodium hydroxide Serum in ml 0.2 Mix read the absorbance at 405nm against blank and extrapolates on the standard graph for the value of GGT in the serum sample. This test is also performed by visible kinetic method. • The normal range according to this method is: Male: 3 to 38 IU Female: 5 to 25 IU • Procedure: The substrate and all reagents are same as mention above. System parameters are: Wavelength: 405 nm Cuvettes: 1 cm light path Flow cell temperature: 300c Tests Substrate in ml 1.5 serum in ml 0.1 Mix well and read initial absorbance change per minute and calculate as follows. • Calculations: 1616 ×mean absorbance /min Questions for practice • • • • Write the important functions of the liver. What is the importance of liver function tests? Which tests are included in the LFT? Write the clinical significance of the following tests. a) SGOT b) SGPT c) GGT Clinical Pathology and Biochemistry : 93 Chapter 2 : Renal function tests • • • • Learning Objectives: To study the different functions of the kidney. To know about the different pathological conditions of renal. Different functions of the kidney : 1) Excretory Function: undesirable end products of metabolism. Formation of urine 2) Endocrine Function: Kidneys produce erythropoietin, renin and prostaglandin.. Production of Vitamin-D3: 3) Regulation of ionic components of blood: e.g. Na+, K+, Ca++, Cl-, HPO4- ions Temperature balance, acid base balance The internal environment of the body is regulated by two organs Lungs and Kidney. • Kidney maintain homeostasis by four processes, 1) Glomerular filtrations of blood 2) Selective re-absorption by tubules 3) Certain substances secretion by tubules conservation of bicarbonate ions 4) Urine formation. Due to decrease glomerular filtration rate waste product like urea, creatinine and uric acid level is found to be increased in the blood. • 1. 2. 3. 4. 5. 6. 7. 8. 9. Kidney Function Test: Routine urine Examination Blood Urea Nitrogen Serum Creatinine Serum Uric Acid Serum Electrolytes Blood Gases, Blood pH and Bicarbonate Plasma and urine osmolarity Creatinine clearance Test Urea clearance Test Clinical Pathology and Biochemistry : 94 Fig 4.2.1 Kidney • Urea: In the liver de-amination of amino acids produce ammonia and it is used in the synthesis of urea.Two molecules of ammonia and one molecule of CO2 are converted to urea for each turn of kerb cycle also called urea cycle. Urea is filtered at glomeruli and in 24 hours 20 to 30 grams is excreted in the urine. The normal level in the serum is 10 to 45 mg/dl. • Determination of urea from the given blood samples by diacetyl -monoxime method • Clinical significance: Increased values in pre-renal conditions are D.M., dehydration, severe burns, cardiac failure and high fever. The post renal conditions are enlargement of prostate, stone in urinary tract, tumour of bladder, and the renal conditions are diseases of the kidney. • Introduction The internal environment of the body is regulated by lung and the Kidney. Kidney maintains optimal chemical composition of the body fluids by acidification of urine and by removing metabolic wastes such as urea, creatinine, uric acid etc. Function of kidney consists of four processes: • Filtration of blood plasma by glomeruli, • Selective absorption by tubules of certain substances which are required to maintain internal environment (Homeostasis) • Secretion of certain substances such as creatinine, Urea etc • Exchange of Potassium for hydrogen ions and ammonia for the conservation of bicarbonate. • In renal diseases due to disturbance in the above four process and due to decrease in glomerular filtration rate urea, creatinine tends increased in blood. Clinical Pathology and Biochemistry : 95 De-amination of major amino acid in liver produces keto acids and ammonia. Large quantity of ammonia is converted into urea in liver by Krebs’s cycle. Urea is filtered at the glomeruli and after practical re-absorption it is excreted in urine. • Urea is major containing nitrogen metabolic product of protein catabolism in humans, more than 90% 0f urea is excreted trough kidney. • Method: Di-acetyl monoxime method • Principle: Urea present in the serum sample reacts with dia-cetyl-monoxime in hot acidic medium and in the presence of thio-semi-carbazide and ferric ions to form a pink coloured complex can be measured on a green filter. • Sample: 2-3 ml of blood collected in an EDTA bulb and plasma is separated. • Normal range: Birth to 1 year; 4-16 mg/dl Adults up to 40 mg/dl • Preparation of reagents: Reagent 1: (DMR):0.2 gram of Diacetyl monoxime in 100 ml of Distilled water Reagent 2: (TSC) 40 mg/dl thiosemicarbazide. Reagent 3: (Acidic) 60 ml of H2SO4 (concentrated), 10 ml of ortho-phosphoric acid and 10 ml of 1.0 gram/dl in 1 litre of distilled water. Preparation of working reagent: one part of reagent 1 + one part of reagent 2 + two parts of reagent 3 prepare fresh. 6. Urea Standard: 20mg/dl in 100ml optical density saturated benzoic acid. • Procedure: Pipette in a tube labelled a follow, Test ml Std.ml Blank ml 20mg/dl Working reagent 5 5 5 Serum/plasma 0.1 Standards 0.1 D.W -0.1 Mix the contents of the tube thoroughly and place them in a boiling water bath for 25minutes Cool immediately and measured the O.D against blank at 520nm. • Calculation: Optical density of test × concentration of standard (20) = Optical density of standard • Clinical significance: In the renal dysfunction the urea and creatinine values are important and indicate the severity of the dysfunction. Elevated levels of urea observed in pre-renal and post renal conditions. • Pre renal conditions are D.M, dehydration, cardiac failure, severe burns high fever • Renal conditions are: Diseases of kidneys. • Post renal conditions are; Enlarge of prostates, stones in urinary tracts, tumour of the bladder. Lower values are seen in malnutrition. • Clinical Pathology and Biochemistry : 96 • Creatinine: • Introduction: It is end product of creatine metabolism, is anhydride of creatine. Creatine is present in muscle brain and blood in the free as well as in the form of creatine phosphate. After removal of water molecule from the creatine phosphate the creatinine is formed. During severe exercise creatine phosphate stored in the muscle in converted to ATP by donating its phosphate group. At the same time creatine gets converted to creatinine a waste product of creatine metabolism. Creatinine thus formed is filtered at the glomeruli and secreted by the tubules. Its excretion in urine per 24 hours is 1.5 to 3 grams. In renal diseases the plasma creatinine level increases. Jaffe’s method is used to determine creatinine in the blood sample. • Determination of creatinine by Jaffe’s method: • Principle: Creatinine in the blood sample reacts with the picric acid in alkaline medium. This generates a reddish yellow complex. The intensity of the colour formed is directly proportional to the concentration of creatinine in the blood sample. • Clinical significance: In renal failure the values are found to be increased. The increased value above 2 mg/dl is virtually diagnostic of renal diseases. In mechanical obstruction of urinary tract its values are found to be increased. • Normal range: 0.7 to 1.5 mg/dl • Preparation of the reagents: Reagent 1: picric acid .; Dissolve 10.5 grams of hydrated or 9.5 grams of anhydrous picric acid in to 500ml of hot 80oc D.W and make the final volume 1000 ml using D.W. Reagent 2: 0.75 M NaOH: Dissolve 30 grams in 800 ml Distilled water and make the final volume 1000ml using Distilled Water.. Sulphuric acid: Add 18.8 ml ofpure grade AR in 800 ml of D.W. and make the final volume 100ml using D.W. Sodium Tungstate: Add 50 grams in 500 ml D.W. and dilute to 1000 ml Hydrochloric acid 0.1 N Creatinine standard: 2 mg/100 ml • • Procedure: A) Preparation of protein free filtrates Pipette in a centrifuge tube labelled as follow, Serum in ml Sodium tungstate D.W 2/3 N H2SO4 Test in ml 1 1 1 1 Clinical Pathology and Biochemistry : 97 Mix the content after each addition wait for 10 min., centrifuge and get clear filtrate 2 ml of supernatant is required for the test. • B) Colour reaction: Prepare another set of three test tubes label as follow and make the additions as follow. Prepare fresh reagent 4 parts of picric acid and add 2 parts of sodium hydroxide and use. Test Std Blank D.W 3ml 3ml 3 ml Standard 2 ml Filtrate 2 ml Freshly prepared reagent 1 ml 1 ml 1ml Mix and keep at room temperature for 20 minutes and measure the absorbance at 520nm green filter against blank. • Observations: Specimen O.D.at 520 nm Test standard • Calculations: (O.D. of test ÷ O.D. of standard) ×1.0 • Uric acid (UA) • Introduction: It is formed from the breakdown of nucleic acids and excreted by kidneys. It is end product of purine metabolism. Two purine Adenine and guanine undergo catabolism and produce Uric acid. It is filtered at glomeruli and partially reabsorbed by the tubules and then excreted in the urine. Hyper-uraemia is a condition when the concentration of uric acid is more than normal value. UA is insoluble in water and the excess Uric acid crystals precipitated and deposited in the tissues and joints. They also deposited in the soft tissue and there is intense inflammatory response. Gout is associated with hyper-uraemia where monosodium urate crystals are precipitated in the joints. The Gout condition arises because of the following reasons: Increase synthesis of purine nucleotide. Renal failure Lactic acid acidosis and ketoacidosis Hypertension Increases tissue breakdown • Determination of serum Uric acid (UA) by Henry- Caraway’s method • Clinical significance: It is end product of nucleoprotein metabolism. It is a low threshold excretory product. Its values are often increased in the Gout, renal failure, leukaemia, and uraemia. HyperClinical Pathology and Biochemistry : 98 uricemia is a condition always associated with the high levels of uric acid in the blood which then get deposited in the joints as sodium urate crystals. • Method: Henry- Caraway’s method • Principle: Uric acid in the protein free filtrate react with phospho-tungstic acid, reagent in the presence of alkaline solution to form blue colour complex which is measured at 660 nm. • Reagents: 10 gm./dl Sodium carbonate Phospho-tungstic acid reagent: 50 gram of phospho-tungstic acid, 40 ml of ortho-phosphoric acid and 400 ml of distilled water mix and reflux for two hours make the final volume 500 ml store at 2 to 8oC dilute 1 ml of this reagent to 10 ml using distilled water and then use. Uric acid standard: 5 mg/dl. De-proteination of the serum sample: De-protonating reagent: 10 g/dl sodium tungstate 50 ml, 2/3 N Sulphuric acid ortho-phosphoric acid one drop , distilled water 800 ml mix and store. Take 5.4 ml of this reagent and 0.6 ml of serum mix and centrifuge use supernatant for the test. • Addition table: Filtrate Standard D.W Sodium carbonate reagent Phospho-tungstic acid reagent Test 3ml 1 ml 1 ml Std 2 ml 1 ml 1 ml Blank 3 ml 1ml 1 ml Mix and keep at room temperature and in dark for 10 minutes and measure the absorbance at 660nm green filter against blank. • Calculations: (O.D. of test ÷ O.D of standard) ×5.0 • Glomerular filtration rate (GFR) • Introduction: This is also known as clearance test which determine the filtrations capacity of the nephrons. Large volume of blood one litre per minute flows through the kidney. Each nephron produces 100 microliter of ultra-filter per day. Each kidney contains one million nephrons. About 125 ml of filtrate is formed in 1 minute i.e. glomerular filtration rate is 125 ml/minute. Glomerular filtrate is an essentially protein free and cell free extracellular fluid. Creatinine clearance gives accurate and useful measure of the glomerular filtration rate and also the excretory capacity of the kidney. This is because creatinine is not reabsorbed by the tubules and blood creatinine values are relatively stable. The clearance of any substance is defined as the number of ml of plasma which contains the amount of that substance excreted in urine in one minute. Clinical Pathology and Biochemistry : 99 This is given by the formula, Clearance = (U V ÷S (p)) × (1.73 ÷ A) Where, A= Surface area of the patients. S (p) = mg/ml of serum creatinine U= mg/ml of urine creatinine 1.73 is standard average surface area of the normal individual. V= ml of urine excreted per minute. Fig 4.2 Functions of kidney • Normal values: Males: 105± 20 ml/min Females: 95 ± 20 ml/min • Clinical significance: In kidney disorder there is marked decrease in the GFR. • Instructions to the patient: Collect the 24 hours urine sample in a plastic container in which thymol is added as a preservative. • Procedure: • Note the age and height of the patient. • Collect the blood sample and perform creatinine determination as prescribe earlier. • Measure the total volume of the 24 hours urine sample and calculate the volume of urine excreted per minute. • Dilute the urine specimen 1:10 using distilled water and Determine the urine creatinine concentration. • Determination of Urine creatinine: Find out the ratio of 1.73/ A from the chart. Calculate the creatinine clearance using following formula: (Urine creatinine÷ serum creatinine) × (Volume ÷Minutes) ×(1.73÷A) Clinical Pathology and Biochemistry : 100 Chapter 3 : Endocrine function test • • • Learning objective: To learn the different hormones secreted by pituitary glands To know the different disease conditions associated with the hormonal changes • Introduction: Endocrine glands are very important they secrets the hormones. These are ductless glands. They catalyse and control metabolic processes of the body. The endocrine system is referring to the collection of cells, glands, and tissues of an organism that secrete hormones directly into the blood stream to control the organisms' physiological and behavioural activities. The endocrine system is in contrast to the exocrine system, which secretes its chemicals using ducts. The word endocrine derives from the Greek words "endo" meaning inside, within, and "crinis" for secrete. • Features of endocrine glands: These are, in general, ductless, vascular and contain intracellular vacuoles or granules storing their hormones. In contrast, exocrine glands, such as salivary glands, sweat glands, and glands within the gastrointestinal tract, tend to be much less vascular and have ducts or a hollow lumen. • The endocrine glands: Pituitary gland , thyroid gland , parathyroid gland, adrenal gland, Islets of Langerhans in the pancreas, ovaries in female, testes in male. Clinical Pathology and Biochemistry : 101 • The pituitary gland : It is also called as adenohypophysis. It is an endocrine gland about the size of a pea and weighing 5 grams in humans. It is a protrusion off the bottom of the hypothalamus of the brain. The Pituitary gland secretes nine hormones that regulate homeostasis and the secretion of other hormones. It is divided in to two lobes anterior and posterior Fig 3.1 :Location of different glands and their hormones • Anterior pituitary lobe (adenohypophysis) Secreted hormone Growth hormone (somatotropin) Abbreviation GH Thyroid-stimulating TSH hormone (thyrotropin) Functions of hormones Stimulates growth and cell reproduction Stimulates Insulin-like growth factor 1release from liver Stimulates thyroxin (T4) and triiodothyronine (T3) synthesis and release from thyroid gland Stimulates iodine absorption by thyroid gland Clinical Pathology and Biochemistry : 102 Adrenocorticotropic ACTH hormone (corticotropin) Beta-endorphin Follicle-stimulating hormone - Inhibits perception of pain FSH In females: Stimulates maturation of ovarian follicles in ovary In males: Stimulates maturation of seminiferous tubules In males: Stimulates spermatogenesis In males: Stimulates production of androgen-binding protein from Sertoli cells of the testes Luteinizing hormone LH Prolactin Stimulates corticosteroid (glucocorticoid and min-eralcorticoid) and androgen synthesis and release from adrenocortical cells PRL MelanocyteMSH stimulating hormone In females: Stimulates ovulation In females: Stimulates formation of corpus luteum In males: Stimulates testosterone synthesis from Leydig cells (interstitial cells) Stimulates milk synthesis and release from mammary glands Mediates sexual gratification Stimulates melanin synthesis and release from skin/hair melanocytes • 1)Growth Hormone: • Functions: 1) Protein synthesis: Increases retention in nitrogen and phosphorus, stimulate protein synthesis. 2) Lipid Metabolism: It is mildly lipolytic. 3) Carbohydrate metabolism: In muscles it antagonizes the effect of insulin. • Normal range: 0 to5 ng/ml • Clinical significance: Plasma GH concentration increased following stress like surgical stress, cold, pain, and exercise Its deficiency in children results in Dwarfism. Acromegaly results from excessive release of GH. Clinical Pathology and Biochemistry : 103 • 2)TSH It is glycoprotein and its release is control by thyrotrophic releasing hormone (TRH) • Functions: It increases thyroid growth and general metabolic activity Including, 1) Glucose oxidation. 2) Oxygen consumption. 3) RNA and Phospholipid synthesis. 4) Iodine uptake regulation. 5) Thyroxin metabolism. • Normal range: Adults: 0 to 10 µIU /ml. • Clinical significances: High values of TSH are found in primary hypothyroidism. In hyperthyroidism the values of TSH are found to be decrease. The determination of values is important in the treatment. • 3) ACTH It is straight chain polypeptide. • Functions: 1) Adrenal function is regulated by ACTH. 2) It increases the synthesis and stimulation of corticosteroids by adrenals. 3) It increases the total protein synthesis. 4) ACTH stimulation results in an increase in mineralocorticoids, glucocorticoids and androgens. 5) It mobilizes prostaglandins in the adrenal. • Clinical significance: Excess production of ACTH produces Cushing’s syndrome. • 4) FSH It is also called as the Gonadotropins. It is glycoprotein. Its secretion is regulated by the hypothalamus secreting FSH releasing hormone. • Functions: 1) Promotes follicular growth and prepare the follicle for the action of LH. 2) In the male it stimulates seminal tubule and testicular growth. 3) It play important role in the early stage of spermatogenesis. 4) It induces the release of LH to enhance the release of estrogen. • Normal range: Male: 2 -25 µIU /ml Female: 4 to 30 25 µIU /ml Postmenopausal Female: 40 to 250 25 µIU /ml in • Clinical significance: Increased values are found in menopause, castration and in seminiferous tubule failure. Decreased levels are found in hypogonadism, anorexia nervosa and in the neoplasms of the testis.] Clinical Pathology and Biochemistry : 104 • 5) LH It is also called as gonadotropins and is a glycoprotein. • Functions: 1) In the female it stimulate the final maturation of the follicle, ovulation. 2) It also stimulates progesterone and estrogens levels. 3) In the male it stimulates testosterone production by testis. • Normal range: Men: 7 to 24 mIU /ml Women: 6 to 30 mIU /ml • 6) Prolactin It is a protein. • Functions: 1) It activates corpus luteum. 2) It stimulates continuous progesterone production by the development of corpus luteum. 3) During pregnancy it stimulates mammary development. 4) It stimulates metabolic changes like growth hormones. • Clinical significance: In women tumours of prolactin secreting cells cause galactorrhea (breast discharge). In men excess prolactin is associated with the breast enlargement and impotence. • Normal range: Male: 5 to 18 ng/ml 1) Female: 6 to 22 ng/ml • Posterior pituitary lobe (neurohypophysis): Secreted hormone Abbreviation From cells Effect Oxytocin Magnocellular neurosecretory cells Uterine contraction Lactation Vasopressin (antidiuretic hormone) neurosecretory neurons Increases water permeability in the distal convoluted tubule and collecting duct of nephrons, thus promoting water reabsorption and increasing blood volume ADH or AVP Oxytocin and anti-diuretic hormone are not secreted in the posterior lobe, merely stored. • 1) Oxytocin Functions: It promotes contraction of the uterine muscles and cells of lactating breast. This help in squeezing of milk. Clinical Pathology and Biochemistry : 105 • 2) ADH Functions: 1) It acts on distal convoluted tubules and collecting tubules of the nephrons of the kidneys increases permeability and re-absorption of water from the glomerular filter increases. 2) When the osmotic pressure of the blood is low the secretion of ADH decreases and reduces the water re-absorption producing diluted urine. • THYROID GLAND Thyroid gland produces 2 hormones Hormone Full form Released for Triiodothyronine Whole T3 body T4 Thyroxine Whole body Functions Both cause an increase in BMR. They play a role in carbohydrate, protein, vitamin metabolism. Increased breakdown of fats to provide energy. Decrease cholesterol level. Necessary for normal development of nervous system, Essential for maturation of embryo and growth and development of embryo’s nervous system. Necessary for normal gonad activity, formation of milk, formation of RBC Required for maintenance of skin’s normal texture. Clinical Pathology and Biochemistry : 106 • TFT : THYROID FUNCTION TESTS 1. T3 (Triiodothyronine) 2. T4 (Thyroxine) 3. TSH (Thyroid stimulating hormones) 4. Free T3 5. Free T4 • Adrenal glands: There are two adrenal gland situated on the upper pole of each kidney, composed of two parts outer part is called as adrenal cortex and inner part is called as adrenal medulla. Adrenal cortex produces three groups of hormones collectively called as corticosteroids. They are as follows, Secreted hormone Effect Glucocorticoids (chiefly cortisol) Stimulates gluconeogenesis Stimulates fat breakdown in adipose tissue Inhibits protein synthesis Inhibits glucose uptake in muscle and adipose tissue Inhibits immunological responses (immunosuppressive) Inhibits inflammatory responses (antiinflammatory) Mineralocorticoids (chiefly aldosterone) Stimulates active sodium re-absorption in kidneys Stimulates passive water re-absorption in kidneys, thus increasing blood volume and blood pressure Clinical Pathology and Biochemistry : 107 Stimulates potassium and H+ secretion into nephron of kidney and subsequent excretion In males: Relatively small effect Androgens (including DHEA and testosterone) compared to androgens from testes In females: masculinizing effects • 1) Glucocorticoids (chiefly cortisol) Cortisol and corticosterone are main glucocorticoids, secreted by stimulation of ACTH. • Functions: 1) Promotion of glycogenesis 2) Promotion of gluconeogenesis 3) Decrease in protein synthesis. 4) Increase in lipolysis. 5) Re-absorption of sodium and water from renal tubules. • Clinical Significance: High concentration of these hormones has anti-inflammatory and immune-suppressive effects. • 2) Mineralocorticoids: Aldosterone is main Mineralocorticoids. The amount of aldosterone produced is totally depending on the blood sodium level. • 1) 2) 3) Functions: Control re-absorption sodium by the renal tubules. Stimulates excretion of the potassium by the renal tubules. It increases the water excretion by the kidney. • Adrenal medulla: The amines dopamine, nor-epinephrine, epinephrine are synthesized in the chromaffin cells of the adrenal medulla. The major product of the adrenal medulla is epinephrine which is a catecholamine derivative of tyrosine and phenylalanine. Stimulation of splanchnic nerve results in the release of catecholamine’s which in association with albumin circulating in plasma. • Functions: Epinephrine is associated With potentiating the condition needed for fight after the initial sympathetic stimulation is by constricting skin blood vessels, increasing metabolic rate, Dilating blood vessels of muscles etc. Clinical Pathology and Biochemistry : 108 • Adrenal medulla: Secreted hormone Effect Fight-or-flight response: • Boost the supply of oxygen and glucose to the brain and muscles (by increasing heart rate and stroke volume, vasodilation, Adrenaline (epinephrine) (Primarily) increasing catalysis of glycogen in liver, breakdown of lipids in fat cells) • Dilate the pupils • Suppress non-emergency bodily processes (e.g., digestion) Fight-or-flight response: • Boost the supply of oxygen and glucose to the brain and muscles (by increasing heart rate and stroke volume, vasoconstriction and increased blood pressure, breakdown of lipids in fat cells) • Increase skeletal muscle readiness. Noradrenaline (norepinephrine) Dopamine Increase heart rate and blood pressure Enkephalin Regulate pain • Hormones of the gonads: The gonads are bi-functional organ which produce germ cells and the sex hormones .The testes produce spermatozoa and testosterone and ovaries produce ova and steroid hormones estrogen and progesterone. • 1)Testosterone: LH stimulates its production. The primary product of testes is Testosterone. • Functions: 1) Spermatogenesis is regulated by FSH and Testosterone 2) It is involved in sexual differentiation, gene regulation, development of secondary sexual organs. • Clinical significance: Lack of testosterone synthesis is called hypogonadism if it occurs before puberty secondary sexual characteristics fail to develop. Primary hypogonadism is due to defective secretion of the gonadotropins. Clinical Pathology and Biochemistry : 109 • Normal value: The secretion rate of testosterone is about 5 mg/dl in normal adult male. • 2) Estrogen: Ovarian hormone synthesized by the follicles and corpus luteum of ovary. • Functions: 1) Sex related physiological functions 2) Development of female sexual characteristics. 3) Growth, development and maintenance of female reproductive system. 4) Maintenance of menstrual cycle. | 5) Increase in the synthesis of many proteins like transferrin, ceruloplasmin in liver. 6) Increase in the HDL fraction. 7) Induce the synthesis of progesterone receptors in the uterus and mammary glands. 8) Induce the synthesis of progesterone receptors in the uterus and mammary glands. • 3) Progesterone: It is synthesize from cholesterol by corpus luteum and placenta. It is synthesize from cholesterol by corpus luteum and placenta. • Functions: 1) Required for the implantation of fertilized ovum and maintenance of pregnancy. 2) It promotes the growth of glandular tissues. 3) It also promote the growth of uterus and mammary glands • Normal Values: Males: under100mg/dl Females: Follicular phase: Below 150 mg/dl Luteal phase: At least 300 mg/dl 1500 to 5000 mg/dl during first trimester • 3) Human Chorionic Gonadotropin (HCG) It is produced by placenta in pregnant females found in blood, milk urine and fetal tissues. • Functions: 1) Support the corpus luteum until the placenta produces amounts of progesterone sufficient to support pregnancy. • Normal values: Present during pregnancy appears soon after the nine days of conception and reaches the peak between 7th and 12th week of gestation. Clinical Pathology and Biochemistry : 110 Questions for practice • What are the different functions of Growth hormones? • Write the functions of the following, a) FSH b) TSH c) Progesterone d) Mineralocorticoids • Write the normal range of the following, • TSH b) oestrogen c) Testosterone) Glucocorticoids • Write the names of adrenal cortex hormone • Write the names of the adrenal medulla hormones • Which amino acid is required in the synthesis of thyroid hormones Clinical Pathology and Biochemistry : 111 Chapter 4 : Lipid profile • • • Learning objective: To learn the important of lipids. To study the risk of elevated levels of lipids • Introduction Lipids are synthesized in the body from dietary fats. Cholesterol, Triglycerides, high density lipoprotein, low density lipoprotein and very low density lipoprotein are commonly measured lipids in the body. Lipids are a group of organic substances, fatty in nature insoluble in water and soluble in organic solvent such as ether, alcohol. • Importance of lipids: Reservoir of high energy, important component of cell membrane, form important constitution of nervous tissue, form insulating and protective coating around certain organ forms lipoprotein in the body and in the form of oil soluble vitamins like A,D E and K they are important dietary source. Cholesterol, Triglycerides are important in the clinical practice because of their association with the cardiovascular diseases. • 1) Cholesterol: Cholesterol is present in all tissues, is a main sterol, is a solid alcohol of high molecular weight and is the initial starting point in many metabolic pathways including vitamin D synthesis, steroid hormone synthesis and bile acid metabolism. • • Source: Animal diets such as meat, egg yolk, fat- dairy product provide bulk of cholesterol. Plant diet also contains cholesterol. It is absorbed by formation of mixed micelle by bile acids. This contains un-esterified cholesterol, fatty acids, phospholipids, mono glycerides and conjugated bile acids. In the absence of bile acid the digestion and absorption of cholesterol and triglycerides is impaired. • Determination of serum cholesterol: • Clinical significance: Elevated levels are found in atherosclerosis, Nephrosis, Diabetic mellitus, Myxedema. Decreased levels are seen in hyperthyroidism, mal-absorption and anaemia. • Method: Watson’s method • Sample: Un-hemolysed serum Clinical Pathology and Biochemistry : 112 • Principle: Cholesterol reacts with acetic anhydride in the presence of glacial acetic acid and concentrated sulphuric acid to form green colour complex intensity of which is directly proportional to the concentration of cholesterol in the sample. • Normal range: 150 to 250mg /dl • Preparation of the reagent: Cholesterol reagent: 5.6 gram of 2,5 dimethyl benzene-sulphonic acid in 200 ml of glacial acetic acid and 300 ml of acetic anhydride. • Reagent 2: Concentrated sulphuric acid • • Reagent 3: Standard cholesterol 200 mg/dl Addition table: Test standard blank Cholesterol reagent in ml 2.5 2.5 2.5 Serum in ml 0.1 Standard in ml -0.1 Distilled water in ml 0.1 Mix well and cool a And then add following reagent concentrated sulphuric acid in 0.5 0.5 0.5 ml Mix and keep incubate at room temperature for 15 minutes, read the absorbance at 575 nm against blank. • Calculations: (O.D. of Test÷ O.D of Standard) × 200 • 2) HDL cholesterol HDL is produced in the liver and intestine. 50% HDL mass is protein, 30% phospholipids and 20% cholesterol. It plays an important role in the cholesterol efflux from the tissue, it has a role in returning cholesterol from the periphery to the liver for removal as bile acids. HDL obtains free cholesterol from the VLDL and chylomicrons which are triglyceride rich lipoproteins. The catabolism of the VLDL and chylomicron totally depend upon the HDL concentration. If there is marked reduction in the HDL there is defect in the triglyceride rich lipoprotein catabolism. • Determination of HDL cholesterol: • Clinical significance: It is also called as good cholesterol important in the catabolism of triglyceride rich VLDL and chylomicron. Lower values of HDL cholesterol indicate high risk of coronary diseases. In the patients with acute myocardial infarction the HDL values are on higher side. Clinical Pathology and Biochemistry : 113 • Normal values: Men: 30 to 60 mg/dl Women: 40 to 70 mg/dl Specimen: Clear serum. Principle: In the presence of Phopho-tungstic acid and magnesium chloride LDL, VLDL and chylomicrons are precipitated. After centrifugation only HDL appears in the supernatant. • Reagents: • Precipitation reagent: Phosphotungstic acid reagent: (PTA) 2.25 gram of Phosphotungstic acid in 8 ml of 1 N NaOH and add 42 ml of distilled water. Magnesium chloride reagent: 2 gram of MgCl2 in 50 ml distilled water. • Cholesterol standard: 100 mg/dl Other reagents are same as the reagents of cholesterol determination • Precipitation: Test serum in ml 0.5 P.T.A reagent in ml 0.05 Magnesium chloride in 0.02 ml Mix well and centrifuge at 3000 rpm for 20 min. Use supernatant for the HDL determination. • • • HDL determination addition table: Test 2.5 0.1 -- Cholesterol reagent in ml Supernatant in ml Standard in ml ( 100mg/dl) Distilled water in ml Mix well and cool a And then add following reagent concentrated sulphuric acid in 0.5 ml standard 2.5 0.1 - blank 2.5 0.1 0.5 0.5 Mix and incubate at room temperature for 15 minutes, read the absorbance at 575 nm against blank. • Calculations: (O.D. of Test÷ O.D of Standard) × 114 • 3) LDL cholesterol: The Catabolism product of VLDL is IDL which is short lived intermediate, partly depleted of triglycerides and continue catabolism of IDL produce LDL. The LDL catabolism takes place in the liver and peripheral tissues and produce free cholesterol which enter in the cytoplasm. Clinical Pathology and Biochemistry : 114 • 4)Triglycerides: • Introduction: Triglyceride is a main form of lipid storage in human which is 95% of fat in adipose tissue. It consists of three chains of fatty acid attached to glycerol backbone. They are transported in the plasma bound to lipoproteins. The main lipoproteins are HDL and LDL. • Determination of serum triglycerides: • Clinical significance: Increased levels of triglycerides cause plasma to have milky appearance. Hyperlipidaemia is excess of lipids like triglycerides which is a risk of myocardial infarction. • Method: enzymatic • Normal range: Up to 150 mg/dl • Reagents: Buffer, enzyme and chromogen: A) 1) Lipoprotein lipase 30 units 2) glycerol kinase 10 units 3) Glycerol phosphate oxidase 5 units 4) peroxidase 5 units 5- Glycerol phosphate in 100 ml of phosphate buffer of pH 7.2 and B) P- chloro-phenol 30 mg/dl Triglyceride standard: 100 mg/dl Prepare by mixing two parts of regent A and one parts of reagent B and use freshly. • Addition table: Working reagent in ml Serum in ml Standard in ml Distilled water in ml Test 1 0.0q -- standard 1 0.01 - blank 1 0.01 Mix and keep incubate at 37oC for 15 minutes, read the absorbance at 520 nm against blank. • Calculations: (O.D. of Test ÷ O.D of Standard) × 100 LDL= Cholesterol – HDL – (Triglycerides ÷ 5) VLDL = Triglycerides ÷ 5 Questions for practice • • What are lipid profile tests? Write the principle of the determination of cholesterol by end point method • What is the clinical significance of cholesterol and triglycerides in the blood? Clinical Pathology and Biochemistry : 115 Chapter 5 : Glucose Tolerance Test • • • Learning objectives: To interpret the Glucose tolerance test in different clinical conditions. To understand the importance of GTT • Introduction: A glucose tolerance test is a medical test in which glucose is given and blood samples taken afterward to determine how quickly it is cleared from the blood. It is a lab test to check how your body breaks down sugar. Alternative name of the test is oral glucose tolerance test. The oral glucose tolerance test (OGTT) has been considered to be the gold standard for making the diagnosis of type 2 diabetes. It is still commonly used during pregnancy for diagnosing gestational diabetes. • Instructions to the patients: Make sure you eat normally for several days before the test. At least three days before the test a high carbohydrate diet has to be followed (230-300 grams per day). All medication and nutritional supplements should be suspended a few days before the test, because they might influence blood glucose metabolism. No meals should be eaten after 8:00 PM on the day before the test. During the test, no eating or smoking is permitted. The diabetes test lasts for 3 hours • Why the Test is performed? Glucose is the sugar which body uses for energy. Patients with untreated diabetes have high blood glucose levels. Glucose tolerance tests are one of the tools used to diagnose diabetes. Above-normal blood glucose levels can be used to diagnose type 2 diabetes or high blood glucose during pregnancy (gestational diabetes). Insulin levels may also be measured. (Insulin is the hormone produced by the pancreas that moves glucose from the blood into cells.) The oral glucose tolerance test is used to screen pregnant women for gestational diabetes between 24 and 28 weeks of pregnancy. It may also be used when the disease is suspected, even though the fasting blood glucose level is normal. Clinical Pathology and Biochemistry : 116 • How the Test is performed? Before the test begins, a sample of blood will be taken by vene-puncture. Ask the patient to collect urine sample at the same time. • The patient will then be asked to drink a liquid containing 75 grams of glucose. Blood sample will be taken again every 30 minutes after you drink the solution simultaneously urine sample of that time interval is also collected. The blood samples are taken up to four times at different time intervals after consumption of the sugar to measure the blood glucose. The test takes up to 3 hours. Throughout the test (the 3 hours between the four blood samples), please observe the following, • No Food • No Drinks (sweetened /unsweetened tea/coffee or any beverage). • No Smoking • No Exercise • Drink plain water • How the patient will feel? Some people feel nauseated, sweaty, light-headed, or may even feel short of breath or faint after drinking the glucose. However, serious side effects of this test are very uncommon. When the needle is inserted to draw blood, some people feel moderate pain. • Risks: Veins and arteries vary in size from one person to another and from one side of the body to the other. Obtaining a blood sample from some people may be more difficult than from others. Other risks associated with having blood drawn are slight but may include: • • • • Excessive bleeding Fainting or feeling light-headed Hematoma (blood accumulating under the skin) Infection (a slight risk any time the skin is broken) • Normal Results: Normal blood values for a 75-gram oral glucose tolerance test used to check for type 2 diabetes in those who are not pregnant: • • • Fasting: 60 -100 mg/dl 1 hour: less than 200 mg/dl 2 hours: less than 140 mg/dl • Procedure for determination of serum /plasma glucose by glucose oxidase method: • Clinical significance: Increased glucose levels may found in diabetic mellitus, hyperthyroidism, hyperpituitarism, adrenocortical hyperactivity .In diabetic treatment overdose of insulin cause hypoglycaemia so to monitor the blood glucose level of the patient it is very important test. Low values of glucose are also found in hypoadrenalism, hypothyroidism. Clinical Pathology and Biochemistry : 117 • Method: Glucose oxidase method • Principle: Glucose contains aldehyde as a functional group which is oxidised by glucose oxidase to give gluconic acid and hydrogen peroxide .The peroxidase enzyme splits hydrogen peroxide into water and oxygen. The liberated oxygen react with 4- amino-phenazone in the presence of phenol to form pink colour compound intensity of which is determined at 530 nm. • Sample: Anti-coagulated blood sample. Fluoride or EDTA blood sample • Reagents: Buffer enzymes: The mixture is created by mixing 2 parts of enzyme reagent and one part of phenol reagent given in the commercial available kit or dissolve as per the instructions given in the manual. • Addition table: Glucose reagent Plasma / Serum Standard 100mg/dl Test 3 ml 0.02 ml - Standard 3 ml 0.02 ml Blank 3 ml - Distilled water - - 0.02 ml Mix and incubate at 370C for 15 minutes and take absorbance at 530 nm. • Calculations: (O.D of test ÷ O.D of Std) × 100 • Normal Results Normal blood values for a 75-gram oral glucose tolerance test used to check for type 2 diabetes in those who are not pregnant: • • • Fasting: 60 -100 mg/dl 1 hour: less than 200 mg/dl 2 hours: less than 140 mg/dl If diabetes is found, it might be wise to extend the test to 6 hours to see if hypoglycaemia occurs after the 5th hour. • What Abnormal Results Mean? Higher-than-normal levels of glucose may mean you have pre-diabetes, diabetes, or gestational diabetes. Between 140 - 200 mg/dl is called impaired glucose tolerance. It may be called "pre-diabetes." It means you are at increased risk of developing diabetes. A glucose level of 200 mg/dl or higher is a sign of diabetes. However, high glucose levels may be related to another medical problem (for example, Cushing syndrome). Clinical Pathology and Biochemistry : 118 • Considerations Factors that may affect the test results: • • Acute stress (for example, from surgery or an infection) Vigorous exercise Several drugs may cause glucose intolerance, including: • • • • • • • • • • • • • • • • • Atypical antipsychotic medications, Beta-blockers (for example, propranolol) Birth control pills Corticosteroids (for example, prednisone) Dextrose Epinephrine Estrogens Glucagon Isoniazid Lithium Pheno-thiazine Phenytoin Salicylates (including aspirin) Thiazide diuretics (for example, hydrochlorothiazide) Triamterene Tricyclic antidepressants Your typical glucose tolerance test curves and interpretation: 75 grams of glucose dissolved in water is taken at the beginning of the test. All of the following tests lasted 6 hours, with blood samples being taken at the beginning, 30 min., 60 min, 120 min, 180, 240, 300, and 360 minutes (0, .5, 1, 2, 3, 4, 5, 6 hrs.) Glucose in the urine might be an indication of diabetes. The average blood glucose level at which glucose is found in urine samples is about 170 or 180 mg/dl. The curves shown below are used to diagnose several blood sugar metabolism disorders. Actual curves may vary from person to person and in the same person from month to month. If the measured values lie within the ranges of the following 'normal curve,' and if no typical symptoms are present, then it is concluded that the person has "good enough glucose tolerance". For the sake of simplicity, the following graph shows only: Normal (Min / Max), Mild Diabetes, Severe Diabetes, and Diabetes with Hypoglycaemia. Find out your results and make a conclusion as follows. Clinical Pathology and Biochemistry : 119 1a. Normal Minimum curve: Time [hours] 0 Blood Glucose min [mg/dl] 80 0.5 1 2 3 4 5 6 90 105 90 80 80 80 80 A "Normal-Min" curve means your pancreas is still in very good shape. Insulin release is strong and sufficient to keep glucose from rising. Keep your pancreas healthy by not stimulating much release of insulin, i.e. eat low carbohydrate meals. Pathologic conditions causing flat or depressed glucose tolerance results: 1b. Normal Maximum curve: Time [hours] 0 Blood Glucose max [mg/dl] 120 0.5 1 2 3 4 5 6 135 160 130 110 100 110 105 The "Normal-Max" response means you are already well started on the road to diabetes. This is "normal" but it is not good. You are aging faster than the minimum possible. Your pancreas is still releasing enough insulin, but maybe 10% to 30% of your beta cells are not functioning. The stream of insulin is not as much as it was before which is the reason why glucose is rising. You should care for the remaining beta cells by not stimulating release of insulin. To prevent the advance to diabetes, pretend that you are already a diabetic, and start to take care of yourself, Cut down drastically on carbohydrates. Clinical Pathology and Biochemistry : 120 2. Curve with mild diabetes (Source: Hypoglycaemia, Dr. P. Airola) Time [hours] 0 Blood Glucose [mg/dl] 115 0.5 1 2 3 4 5 6 145 180 160 120 130 130 130 Your pancreas is already partly shut down... perhaps 40% to 60% of your beta cells are burned out, and the stream of insulin is not enough to lower glucose quickly. Your pancreas is working overtime to bring down glucose. For this reason you are burning beta cells at a faster rate than ever before. The low quality of the insulin is not as good as before, and the long time that insulin is present to bring down your glucose causes you to become resistant to insulin. 3. Curve with severe diabetes (Source: Hypoglycaemia, Dr. P. Airola) Time [hours] 0 Blood Glucose [mg/dl] 200 0.5 1 2 3 4 5 6 235 265 280 300 295 280 270 4. Diabetes and hypoglycaemia (Source: Hypoglycaemia; P. Airola) Time [hours] 0 Blood Glucose [mg/dl] 100 0.5 1 2 3 4 5 6 160 220 160 85 60 50 85 This is a curve of a patient that is both diabetic and hypoglycaemic. Continuous low values (Source: Ortho-molecular, 3, 1988, G.E. Schuite-makers) Time [hours] 0 Blood Glucose [mg/dl] 60 0.5 1 2 3 4 5 6 80 100 60 60 60 60 55 If we plot the time on x axis and blood glucose in mg/dl on y axis, the curve T stays under normal levels during the entire test. A rare tumour, called insulinomia, may be the cause. Pre-hypoglycaemia (Source: Hypoglycaemia, P. Airola) Time [hours] 0 Blood Glucose [mg/dl] 90 0.5 1 2 3 4 5 6 115 140 100 85 80 70 75 If we plot the time on x axis and blood glucose in mg/dl on y axis, the curve is typical for a pre-stage of hypoglycaemia. However, a range of mild symptoms may be present at this stage. A 3-hour GTT would not have been long enough to diagnose this type of hypoglycaemia. Clinical Pathology and Biochemistry : 121 Mild hypoglycaemia (Source: Hypoglycaemia, P. Airola) Time [hours] 0 Blood Glucose [mg/dl] 80 0.5 1 2 3 4 5 6 120 80 60 80 75 80 80 If we plot the time on x axis and blood glucose in mg/dl on y axis, the curve represents a mild form of hypoglycaemia. Within the hour, the blood glucose level drops to normal value. During the second hour, the value is far too low; this is typical in case of reactive hypoglycaemia. Consequently, the curve rises until the normal value is reached. Questions for practice : • Define GTT & types • Write its procedure • Write its significance • What is GTT? Explain with the help of diagram a normal response of GTT. • Explain the hyperglycaemia with the help of GTT. • Write the main instruction given to the patient come for GTT test. Clinical Pathology and Biochemistry : 122 Chapter 6 : Amylase • • • Learning Objectives: To learn the mode of action on salivary amylase. To determine the amylase from serum sample. • Introduction: These are group of hydrolase enzyme which hydrolyses, splits starch and glycogen i.e. polysaccharides. Amylase was originally referred as diastase. Two types of enzyme 1) Alpha amylase present in human tissues. 2) Beta amylase. Amylase in the human serum is a small molecule with the molecular weigh 55000 to 60000 so that they pass through the glomeruli of the kidneys and so normally found in the urine. • Mode of action: Amylase attack on alpha 1-4 linkage in a random manner anywhere along the long polyglycan chain and chains are broken down into dextrin, maltose and some glucose units. It has optimum pH at 6.9 to 7 and maximum activity at 370C also found to be active at 500C. Its activity increases in the presence of ions such as chloride, bromide nitrate and chlorate. When linear chain react with iodine it forms deep blue colour and when such chain get fragmented and react with iodine solution there is loss of deep blue colour according to the number of chains, fragments present in the sample. • Determination of amylase: Method: Colorimetric (Amyloclastic, iodometric) • Clinical significance: The pancreatic amylase concentration gives idea about the disease stage of pancreas. So serum amylase values are very important in the diagnosis of disease of pancreas and in the investigation of pancreas function. In acute pancreatitis a transient increase occurs within 2 to 12h of the onset. Tumours of lungs and ovary can produce high level of amylase. Salivary gland lesions caused by infection surgery, irradiation, and tumour shows high levels of serum amylase. • Clinical Pathology and Biochemistry : 123 Fig 6.1 physiology of pancreas • Principle: Amylase in the serum sample reacts with the starch. Starch is a substrate for amylase. Starch first react with iodine reagent and give deep blue colour. After addition of amylase i.e. serum sample it acts on the substrate starch and produces dextrin, maltose as end product. These end products react with the iodine reagent and decrease in the blue colour observed. The disappearance of the blue colour is directly proportional to the amylase concentration in the sample. Normal range: Up-to 400 caraways unit or 0 to 90 IU • • Caraways unit: Caraway defined the enzyme unit as the amount of enzyme that will hydrolyse 10 mg of starch in 30 minutes to a colourless stage. Reagents: Buffered substrate: Add 2.66 gram disodium hydrogen phosphate, 0.86 gram benzoic acid, 0.04 gram starch in 100 ml distilled water Colour reagent: Mix 0.35 gram potassium iodate, 4.5 gram of potassium iodide and 0.9 ml concentrated hydrochloric acid in 100 ml distilled water and before use dilute 1:10 ml using Distilled water. • Clinical Pathology and Biochemistry : 124 • Procedure: Buffered substrate in ml Keep at 37oC for 5 min. Serum in ml Working colour reagent in ml Serum in ml Distilled water in ml Mix neatly Test 2.5 0.1 2.5 20 Blank 2.5 2.5 0.1 20 Read the intensities against blank at 660 nm. • Calculations: (O.D. of blank-O D Of test) ------------------------------------------------ × 4oo O.D of blank • • • Questions for practice What is amylase explain the mode of action of amylase in details. Write clinical significance of amylase Clinical Pathology and Biochemistry : 125 Unit 5 : Principles of analytical technique Learning objectives • Basic steps in analytic chemistry • Titrimetry • Photometry • Electrochemistry • Immunochemistry • Separation& analysis of organic compounds • To get the knowledge of basic steps in analytical technique. • To identify the separate component from the mixture. • To learn about electrophoresis. • To learn the applications of RIA and EIA. • Application of analytical techniques in clinical biochemistry • Introduction: Basic analytical techniques used in clinical biochemistry are Photometry which includes colorimetry, absorption photometry, spectrophotometry, fluorometry and flame emission photometry. Immunochemistry is used for analysis of micro quantities of drugs, toxic compounds and hormone assay. Electrophoresis is used for the separation of closely related compounds. • Basic steps in analytical chemistry: The four basic steps in analytical chemistry are Specimen processing, chemical reaction, standard comparison and calculation as per the formula. Principles of Analytical Techniques: A) Tritrimetry B) Photometry: 1. Electrical Photometry- a.Colorimeter b. Spectrophotometer 2. Flame photometer 3. Flurometry 4. Turbidometry 5. Nephelometry 6. Refractometry C) Electrochemistry 1. Potentiometry- pH meter 2. Coulometer- Chloridometer D) Immunochemistry 1. RIA 2. ELISA E) Separation of Organic compounds 1. Chromatography 2. Electrophoresis Clinical Pathology and Biochemistry : 126 Application of analytical techniques : A)Titrimetry: • What is titration? • Titration is volumetric technique by which concentration of unknown solution (acid or base) is determined by reacting with neutralizing solution of known strength (standard). The end point of neutralization is marked by change of colour of an indicator added to titrating system. • : Uses Used in the preparation of acid base reagent, in determination of titrable acidity of gastric juice, titrimetric analysis of calcium, chloride, bicarbonate for e.g. calcium determination EDTA calcium binder is titrant fluorescent dye Calcein is indicator. In the determination of chloride Schales and Schales method of titration is used in which mercuric nitrate as titrant diphenyl carbazone as the indicator 0.1 N sodium hydroxide is used as titrant with Topfer’s reagent to titrate gastric juice. It is used in the preparation of acid-base reagents in determination of acidity of gastric juice, titrimetric analysis of calcium chloride, bicarbonate. • B) Photometry: • Principle of absorption photometry: It is based on physical laws of light. Light measurement, most common technique use in clinical labs. The principle is based on physical laws of light where intensity of absorbed or emitted light is measured. If a pencil of light is allowed to pass through the coloured solution some amount of light is absorbed while rest is transmitted from it. The amount of light absorbed is proportional to the nature of the solution, the concentration of the solute or colour and the distance of the path of light. This is known as Beer’s law expressed as, A= a b c Where A= absorbance , a = extinction coefficient this is constant for the same analyte and b is distance of the path of light constant 1 cm and c is concentration. Lambert’s Law: Optical density is directly proportional to the path of light where diameter of the cuvette is 1 cm. A c A is inversely proportional to the T (Transmission). More absorbed means less transmitted and relationship is given as A= 2 – log% T. The photo- detector system measure transmitted light and should be first converted to absorbance to calculate the concentration of unknown. Physical properties of light its source and quality is very important in photometry. • Physical properties of light: The visible range of the spectrum covers from 700 nm to 400 nm and beyond the red end of 700 nm are the infrared and heat waves. The wavelength below 400 nm are in the UV region carry high energy deep penetrating actions and so harmful for biological objects. • Source of light: In colorimeter tungsten lamp is used which emits continuous spectrum of light in the visible range 420- 76o nm. • Quality of light: It is regulated by monochromator in the case of spectrophotometer which allow to select one specific wavelength of light to pass through the eat solution. The monochromator may be a prism or a diffraction grating .Special coloured filters are used in colorimeter each has the limitation of Clinical Pathology and Biochemistry : 127 providing a range of wavelengths near the desire wavelength. The colour of the filter is complementary to the colour of the solution to be measured or analysed. • Component of a spectrophotometer/ colorimeter: Fig 5.1 Components of photometry • Light source: It provide radient energy and is a tunsten lamp and hydrogen lamp for the UV and lower visible range. • Monochromator: It provide desired quality of light.In colurimter filters are used in spectrophotometer diffraction gratting is uesd. • Slit: The light from the monochromator is passed through a narrow slit that improves the quality of light by chossing a single wavelength found in all spectrophotmeter. • Cuvette: Are round or rectangular , holds the solution, must be optically trasnperent, scrupulously clean, devoid of any scratch and free from contamination. The optical path within the cuvette is always 1 cm. Glass cuvettes for spectrophotometer and quartz cuvette for uv radiation. It holds the solution may be blank which is used to set zero absorbance. The solution may be standard that is of known concentration within a limit .In the control solution the concentration of the analyte is known and the test solution is a solution into which we add a specimen to develop colour. • Photodetector: It generate electric current when light falls on it and transmitted to galvanometer .The needle of galvanometer deflects as a consequence of the elctric impulse and is directly proportional to the light intensity. The basic technique of calorimetric analysis is first Chemical reactions; second the development of colour, physical measurement and calculations as per the formula. Clinical Pathology and Biochemistry : 128 • 2) Flame Photometer : • Principle The solution under test is passed as fine spray to non-luminous flame. In flame solution evaporates and salt dissociates into electrons. Electrons are unstable and produce excess energy in form of characteristic light. The intensity of light is measured by photo detector. • Uses 1. Determination of Na 2. Determination of K 3. Determination of Chloride 4. Determination of Magnesium 5. Determination of Phosphorus 6. Determination of Lithium • 3) Fluorometry : • Principle Fluorimeter is based on principle of measuring intensity of emitted light due to fluorescence. A fluorescent compound is capable of absorbing light of higher energy and emits light of lower energy. Uses: This method is highly sensitive. It is used in toxicology lab. It is used in analysis of hormones. Disadvantages: High cost and limited use. • 4) Turbidometry : • Principle This is classified under absorption photometry. The radiant energy is intercepted by solid particle which are in state of suspension in liquid medium. Uses: 1. Protein determination in CSF 2. Estimating bacterial population before running antibiotic sensitivity test. • 5) Nephelometry: • Principle: It is similar to that of turbidometry. It measures the intensity of radiation scattered by suspension. Use: If antigen antibody complex forms a fine suspension nephelometry is applied for quantifying immunochemical reaction. • C) Electrochemistry: • Definition It is based on quantitative measurement of electrical energy. Clinical Pathology and Biochemistry : 129 • Electrochemical technology: (Electrochemistry) In this method we study flow of current i.e. Amperometry, potential difference between electrodes i.e. Potentiometry and Measurement of PH. Ion selective electrodes selectively measure a particular ion in the presence of other ions. One electrode contains a known concentration of the ion to be measured is called as reference electrode. The difference between the concentration of ions in the reference electrode and the ions in the unknown solution causes to develop an electrical potential which is measured in voltage and get converted into numbers by microprocessor. Technology of ion selective electrodes is used for measuring electrolytes. Blood gases and PH are determined electrochemically, Determination of PH and Pco2 done by the potentiometric method. • 1) Potentiometry • Principle It measures potential difference (force) between two electrodes. Uses: 1. Determination of PH and Pco2 • 2) Coulometry: • Principle In this method total amount of electricity consumed is measured. Hence the term coulometry is related to coulombs (quantity of electricity) • Faraday’s Law: Law of electrolysis Q=IxT Q is the quantity of electricity consumed (coulumb’s) I is the intensity of electric current T is time in seconds Use: 1. Determination of Chloride (Chloridometer). • D) Immunochemistry: • Definition Application of immunological principles of antigen-antibody binding in analytical chemistry is called as immunochemistry. • Applications of techniques: 1. RIA: Radio-Immune-Assay a. solid phase RIA (sandwich method) b. liquid phase RIA (competitive protein binding) 2. ELIA: Enzyme Linked Immunoassay 3. ELISA: Enzyme Linked Immuno-sorbent assay 4. Fluoro immunoassay 5. Nephelo-metric immunoassay • Uses: 1. Detection of HAA (Hepatitis associated antigen) 2. Hormone assays – Insulin, T3, T4 3. Drug assay, Drug monitoring 4. In toxicology Clinical Pathology and Biochemistry : 130 • Advantages: Analyte present in specimen are in extremely small quantity can be detected with accuracy while no other method can be applied. • Principle: To allow “tagged” antigen or antibody to undergo binding and separation of antigen-antibody complex Degree of binding of tagged antigen or antibody is quantitatively measured. • 1) RIA: Radio Immunoassay • Principle: 1. In this method radioactive iodine –I13 is tagged which emits gamma radiation.. Amount of radioactivity emitted is measured by scintillation counter. • Advantage: 1. It is very sensitive, test 2. Large numbers of samples detected • Disadvantages of RIA 1. Potential radioactive hazard --- Cancer 2. Require expensive equipment 3. It has limited shelf life due to radioactive decay. Uses: Same as immunochemistry Types: 1. Solid phase RIA 2. Liquid phase RIA • Solid phase RIA (Sandwich method) : 1. Non-radioactive antibody is attached to solid surface (polystyrene beads). Antibody binds with antigen in patient’s serum. 2. Buffer wash---- removes free antigen. 3. Antigen-antibody complex + radioactive antibody forms sandwich. 4. Buffer wash- removes free radio-actives antibodies. 5. Sandwich complex measured in scintillation counter. • Liquid phase RIA: Competitive protein binding In this method liquid medium is taken Ex. Competition between Antibody , Radioactive antigen, Non radioactive antigen (Anti insulin)Binding of radioactive antigen is inversely proportional to amount of antigen present in the serum. Method used in diagnosis of patients. • 2) ELISA: • Enzyme-linked immune-sorbent assay (ELISA): Principle: In ELISA, a liquid sample is added onto a stationary solid phase with special binding properties and is followed by multiple liquid reagents that are sequentially added, incubated and washed followed by some optical change like colour development by the product of an enzymatic reaction in the final liquid in the well from which the quantity of the analyte is measured. • Advantages 1. Less expensive 2. Long shelf life 3. Require less expensive – colorimeter Clinical Pathology and Biochemistry : 131 • Disadvantages: Not as sensitive as RIA • Types: 1. Solid phase ELISA 2. Liquid phase ELISA • Uses: 1. HCG hormone in pregnancy 2. HIV, Hepatitis B, HIV Virus determination. 3. Hormones determination. 4. To determine the presence of any drug in the blood sample. Separation of compounds immunoassay: • Introduction: An immunoassay is a biochemical test that measures the presence or concentration of a macromolecule in a solution through the use of an antibody or immunoglobulin. Defence system in our body develops antibodies as a result of foreign substances pathogen enter in the body. The antibodies generated react with antigens and make them inactive. • Principle: The antigen and antibodies react and produce agglutination reaction. It Consist of, • 1) Radial immune-diffusion. • 2) Enzyme immunoassay. • 3) Radioimmunoassay. • 1) Radial immune-diffusion: On a slide agar layer is prepared which contain antihuman globulin or Ig G (or antihuman Ig A or Ig M). It is diffused throughout the agar. The agar contains small wells. One well is filled with the sample second well is filled with a standard of a known amount of IgG. Incubate the plate for several hours. The IgG in a serum sample or standard diffuses out of the well into agar it react with the anti IgG in the agar and forms a white ring of precipitation around the well. The diameter of the ring is proportional to the concentration of the IgG in the sample. Fig 5.2 Radial immune-diffusion showing precipitation reactions Clinical Pathology and Biochemistry : 132 • 2) Enzyme immunoassay: EIAs use to detect antigen or antibody present in the sample. • Principle: Immunoassays rely on the ability of an antibody to recognize and bind a specific macromolecule in what might be a complex mixture of macromolecules. In immunology the particular macromolecule bound by an antibody is referred to as an antigen i.e. antigen – antibody complex formation. The area on an antigen to which the antibody binds is called an epitope. In some cases an immunoassay may use an antigen to detect for the presence of antibodies, which recognize that antigen, in a solution. In other words, in some immunoassays, the analyte may be an antibody rather than an antigen. Immunoassays involve chemically linking antibodies or antigens with some kind of detectable label. • Procedure: Four steps 1) commercially available antigen coated beads are taken. 2) Serum specimen is added and incubated. A patient’s serum may have a primary antibody against the sought and get bind to is forming antigen antibody precipitation. 3) Washing steps: Washing take away the non-attached antibodies on the bead. 4) Secondary antibody linked with appropriate enzyme is antihuman globulins which reacts with any human antibody and get stuck to the bead. 5) Washing removes unlinked secondary antibodies. 6) Appropriate amount of substrate is added to react with the enzyme present on secondary antibody and form colour which is proportional to the amount of primary antibody present in the patient’s sample. Quantitative estimation is possible using Elisa reader. Fig 5.3 Basic steps of ELISA Fig: Diagrammatic representation of basic steps of Enzyme immunoassay Clinical Pathology and Biochemistry : 133 Result: ELISA run on micro-plate reader and read on ELISA reader for absorption. Fig: 5.4 ELISA wells showing colour reaction • 3) Radio -immunoassay: Radioimmunoassay (RIA) is a very sensitive in vitro assay technique used to measure concentrations of antigens (for example, hormone levels in the blood) by use of antibodies. As such, it can be seen as the inverse of a radio-binding assay, which quantifies an antibody by use of corresponding antigens. The RIA technique is extremely sensitive and extremely specific, requiring specialized equipment, it remains among the expensive methods to perform such measurements. It requires special precautions and licensing, since radioactive substances are used. • Procedure: To perform a radioimmunoassay, a known quantity of an antigen is made radioactive, frequently by labelling it with gamma-radioactive isotopes of iodine, such as 125-I, attached to tyrosine. This radiolabelled antigen is then mixed with a known amount of antibody for that antigen, and as a result, the two specifically bind to one another. Then, a sample of serum from a patient containing an unknown quantity of that same antigen is added. This causes the unlabelled (or "cold") antigen from the serum to compete with the radio-labelled antigen ("hot") for antibody binding sites. As the concentration of "cold" antigen is increased, more of it binds to the antibody, displacing the radio-labelled variant, and reducing the ratio of antibody-bound radio-labelled antigen to free radio-labelled antigen. The bound antigens are then separated from the unbound ones, and the radioactivity of the free antigen remaining in the supernatant is measured using a gamma counter. Using known standards, a standard curve can then be generated which allows the amount of antigen in the patient's serum to be derived. The radio- activities for each of a series of known concentrations of standards are used to derive terms for an equation, often sigmoidal curve fit. Diagrammatic representation of the procedure is as follows. Clinical Pathology and Biochemistry : 134 Fig.5.5 Procedure of Radioimmunoassay Clinical Pathology and Biochemistry : 135 Fig 5.6 Radio-Immunoassay • B) Chromatography: • Definition: It is a technique by which a mixture of organic compounds carried in mobile phase (liquid or gas) is separated into its constituents on stationery phase. • Types: 1. Paper chromatography – PC 2. Gas Liquid chromatography – GLC 3. Thin layer chromatography – TLC 4. Column Chromatography – CC 5. High pressure Liquid chromatography – HPLC • Uses: 1. Separation of proteins into pre-albumin, albumin, globulin – ,( 1, 2), , 2. Separation of Lipo-Proteins: -Lipoproteins-HDL, Lipo-prtoein – LDL, Chylomicron –VLDL 3. Separation of sugars – Glucose, Lactose, Fructose, Maltose in infants to identify genetic defect. 4. Separation amino acids: Genetic defect- Leucine, Valine, Glycine etc. Clinical Pathology and Biochemistry : 136 • Procedure of Paper Chromatography: 1. Take Whatman No.1 paper 2. Put markings for application of spots 8 cms apart 3. Take 10 ml test solution & load on spot and dry it. 4. Hang paper in tank, run solvent to flow down on paper overnight 5. Dry paper, Spray spotting compound on paper. Dry paper. 6. Examine unknown components by comparing with known spot. • 1) Affinity chromatography: It is used in the haemoglobin A1C also called glycosylated Haemoglobin determination, purification of biological molecules and other macromolecules. • Principle: The principle of affinity chromatography is that the stationary phase consists of a support medium (e.g. cellulose beads) on which the substrate (or sometimes a coenzyme) has been bound covalently. Ligands attached to the matrix made up of an inert substance bind to the desired molecules within the solution to be analysed and form a permanent bond while all other non-reacted molecules are eluted. This leaves the desire product link to the column. Further column is washed using desire solution and pure sample is recover. That is once the other proteins have all been eluted, the bound enzyme(s) can be eluted in various ways: • By increasing the ionic strength of the buffer, e.g. with a sodium chloride gradient, so weakening interactions between the enzyme and the immobilised substrate • By changing the pH of the buffer, again weakening interactions between the enzyme and the immobilised substrate • By adding a high concentration of substrate (or a substrate analogue) to the elution buffer, so that there is competition between the free and immobilised substrate for the enzyme protein. Fig: 5.7 Affinity chromatography principle Clinical Pathology and Biochemistry : 137 For example in the determination of haemoglobin A1c blood specimen containing HbA1c is added in the column which get attach to the ligand .The impurities are removed. The ligate HbA1c is then finally released by desorption for its measurement by changing the pH of the medium Fig. 5.8 Binding and non binding in affinity chromatography Fig 5.9 Elution Clinical Pathology and Biochemistry : 138 • C) Electrophoresis: • Introduction: Method of separating mixture of the organic compounds such as haemoglobins, proteins, iso-enzymes is useful in the diagnosis of hemoglobinopathies. • Definition The term electrophoresis describes migration of charged particle under influence of electrical field. • Principle: 1. Many important biological molecules such as amino acids, peptides, proteins. Nucleotides, nucleic acid, haemoglobin, lipoproteins possess ionizable groups. 2. Therefore at any given pH, they exist in solution as electrically charged either as anions or cations. 3. Under the influence of electrical field, these charged particles migrate to electrodes. Cations --- Cathode (Negative electrode) Anions --- Anode (Positive electrode) • Factors affecting migration of charged particles: Charge on particle, Time, applied Voltage, Distance between electrodes , Ionic strength of buffer PH of buffer, Size of molecule, Molecular weight of particle. • Types of electrophoresis according to support media: The separation of various components in a sample requires supporting media; 1. 2. 3. 4. • 1. 2. 3. 4. Paper – Whatman filter paper Agar and agarose Cellulose acetate Polyacrylamide Uses: Separation serum proteins into pre-albumin, albumin, globulin- , , , Separation of lipoproteins into HDL, LDL, VLDL Separation of haemoglobin- Hb A-Normal, Hb F-Thalassemia, Hb S-Sickle cell anaemia Separation of iso-enzymes, creatinine kinase CK MM- Muscle disorder CK MB- Hear disorder CK BB- Brain disorder • Principle: Electrophoresis is a separations technique that is based on the mobility of ions in an electric field. Positively charged ions migrate towards a negative electrode and negatively-charged ions migrate toward a positive electrode. For safety reasons one electrode is usually at ground and the other is biased positively or negatively. Ions have different migration rates depending on their total charge, size, and shape, and can therefore be separated. • Components: An electrode apparatus consists of a high-voltage supply, electrodes, buffer, and a support for the buffer such as filter paper, cellulose acetate strips, polyacrylamide gel, or a capillary tube. After a separation is completed the support is stained to visualize the separated components. Clinical Pathology and Biochemistry : 139 Fig 5.10 Components of electrophoresis • Procedure: In case of protein electrophoresis a serum spot is applied on the agarose gel incorporated on slide. Whatman no.1 filter paper strips are used to make sufficient to contact the glass slides on the buffer tank full with vernol buffer. Put on the power supply set voltage to 200V for five slides and run for three hours. Remove the slides and stain with the help of protein stain dye (Ponceau S.) for one hour. De-stain it. • Result: As the electric current passes through the sample different proteins albumin, globulins, alpha1 alpha 2 beta gamma migrates at different rates based on the charge. Albumin has highest rate of migration and gamma globulin has slowest rate of migration. Fig 5.11Protein electrophoresis separation of protein Clinical Pathology and Biochemistry : 140 Fig 5.12 Protein electrophoresis result graphical representation Electrophoresis of haemoglobin is done to identify the abnormal haemoglobin. Clinical significance of haemoglobin electrophoresis: When a mixture of proteins subjected to an electric field the different proteins move with different velocities towards anode at pH 8.9.The rate of migration depend upon the molecular weight and size of various protein. Haemoglobin is a conjugated protein .The normal and abnormal haemoglobin migrates towards anode at different rates and enables to understand. • 1. 2. 3. 4. 5. 6. 7. Basic requirements of electrophoresis: Power supplier Buffer tank fitted with electrodes Buffer Fixative Staining solution De-staining solution De-sensitometer • General methods of electrophoresis: 1. 2. 3. 4. 5. 6. 7. 8. 9. Equal quantities of buffer are placed in cathode and anode compartments. Sample is layered on supporting medium. Supporting medium is connected to buffer by paper wicks. Electrical current is passed by power pack by adjusting voltage and current. After electrophoretic run, supporting medium is placed in fixative for 10 minutes. Staining of supporting medium. De-staining of supporting medium. Place in fixative for 10 minutes. Drying, then stained supporting medium is scanned by densitometer. Clinical Pathology and Biochemistry : 141 Questions for practice • Write the application of Enzyme immunoassay. • What is the principle of Affinity chromatography? • What is the principle of Electrophoresis. • What is Radial Immuno-diffusion? • What is the application of RIA? • Describe the procedure of EIA. • Define Photometry , Titrimetry, Chromatography, Electrophoresis. Clinical Pathology and Biochemistry : 142