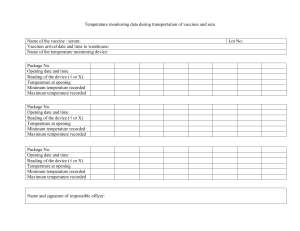
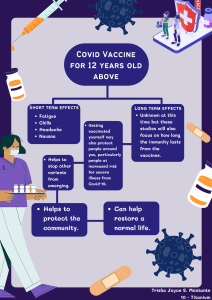
Safety and reactogenicity of the BNT162b2 COVID-19 vaccine Development post-marketing surveillance and real-world data
advertisement

Human Vaccines & Immunotherapeutics ISSN: (Print) (Online) Journal homepage: www.tandfonline.com/journals/khvi20 Safety and reactogenicity of the BNT162b2 COVID-19 vaccine: Development, post-marketing surveillance, and real-world data Frank van den Ouweland, Nicola Charpentier, Özlem Türeci, Ruben Rizzi, Federico J. Mensa, Claudia Lindemann & Shanti Pather To cite this article: Frank van den Ouweland, Nicola Charpentier, Özlem Türeci, Ruben Rizzi, Federico J. Mensa, Claudia Lindemann & Shanti Pather (2024) Safety and reactogenicity of the BNT162b2 COVID-19 vaccine: Development, post-marketing surveillance, and real-world data, Human Vaccines & Immunotherapeutics, 20:1, 2315659, DOI: 10.1080/21645515.2024.2315659 To link to this article: https://doi.org/10.1080/21645515.2024.2315659 © 2024 The Author(s). Published with license by Taylor & Francis Group, LLC. Published online: 26 Feb 2024. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=khvi20 HUMAN VACCINES & IMMUNOTHERAPEUTICS 2024, VOL. 20, NO. 1, 2315659 https://doi.org/10.1080/21645515.2024.2315659 REVIEW ARTICLE Safety and reactogenicity of the BNT162b2 COVID-19 vaccine: Development, post-marketing surveillance, and real-world data Frank van den Ouwelanda, Nicola Charpentierb, Özlem Türecic, Ruben Rizzid, Federico J. Mensae, Claudia Lindemannf, and Shanti Patherg a Medical Safety and Pharmacovigilance, BioNTech, Mainz, Germany; bRisk Management, BioNTech, Mainz, Germany; cBioNTech, Mainz, Germany; Global Regulatory Affairs, BioNTech, Germany, Germany; eClinical Development, Infectious Diseases, BioNTech, Mainz, Germany; fNon-Clinical Safety, BioNTech, Mainz, Germany; gGlobal Medical Affairs, BioNTech, Mainz, Germany d ABSTRACT ARTICLE HISTORY The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to urgent actions by innovators, vaccine developers, regulators, and other stakeholders to ensure public access to protective vaccines while maintaining regulatory agency standards. Although development timelines for vaccines against SARS-CoV-2 were much quicker than standard vaccine development timelines, regula­ tory requirements for efficacy and safety evaluations, including the volume and quality of data collected, were upheld. Rolling review processes supported by sponsors and regulatory authorities enabled rapid assessment of clinical data as well as emergency use authorization. Post-authorization and pharmacov­ igilance activities enabled the quantity and breadth of post-marketing safety information to quickly exceed that generated from clinical trials. This paper reviews safety and reactogenicity data for the BNT162 vaccine candidates, including BNT162b2 (Comirnaty, Pfizer/BioNTech COVID-19 vaccine) and bivalent variant-adapted BNT162b2 vaccines, from preclinical studies, clinical trials, post-marketing surveillance, and real-world studies, including an unprecedentedly large body of independent evidence. Received 6 November 2023 Revised 19 January 2024 Accepted 3 February 2024 Introduction Following the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an urgent need to develop vac­ cines to counter the rapidly spreading pandemic was quickly recognized. While maintaining rigorous clinical trial stan­ dards, multiple stakeholders collaborated to expedite vaccine development, authorization, and roll-out.1 Vaccine clinical development timelines were compressed through leverage of existing research on previous coronavirus outbreaks, the utilization of novel vaccine technologies with shorter design-to-production times, increased funding and collaboration, at-risk investment in commercial production from manufacturers prior to approval, and the use of accel­ erated regulatory procedures and expedited review timelines.1–3 In addition, high infection rates and participant willingness to take part in clinical trials led to more rapid enrollment and study completion, when compared with stan­ dard vaccine development timelines. Pre-existing manufac­ turing processes for messenger RNA (mRNA) vaccines enabled rapid production and scale-up.1 Subsequently, devel­ opment and commercialization timelines were much shorter compared with other vaccines, such as influenza, while every step of the pathway required for regulatory approval was fulfilled (Figure 1).2 The clinical development programs for coronavirus disease 2019 (COVID-19) vaccines, such as BNT162b2, the focus of this review, generated a large amount of safety data. The initial CONTACT Shanti Pather Shanti.Pather@biontech.de KEYWORDS SARS-CoV-2; safety; reactogenicity; vaccine development; postmarketing surveillance; realworld studies approvals were based on compelling efficacy and short-term safety data (up to 2 months follow-up post-primary schedule for BNT162b2, in line with regulatory guidance).5 Variations of these approvals to include a booster dose were based on 2.6 months follow-up post-BNT162b2 booster.6 After authoriza­ tion, vaccine manufacturers continued clinical trials and col­ laborated with regulatory authorities and other organizations for post-marketing pharmacovigilance activities to monitor longer-term safety. The quantity and breadth of this postauthorization safety data quickly surpassed that generated from clinical trials. This was supported by real-world data from countries initiating mass vaccination programs,1 as well as independent clinical and real-world trials in special popula­ tions and with different vaccination regimens, which con­ firmed the safety profile observed in clinical trials, allowed analysis of real-world practice patterns, and created an unpre­ cedented level of transparency.7 The ongoing emergence of new variants of SARS-CoV-2 has led to further generation of safety data for mRNA vaccines, as booster doses and variant-adapted bivalent vaccines have been developed and brought to the market. Here, we review safety and reactogenicity data for the BNT162 mRNA vaccine candidates, including BNT162b2 (Comirnaty, Pfizer/ BioNTech), from preclinical studies, clinical trials, postmarketing surveillance, and real-world studies, as well as from studies of booster doses and variant-adapted BNT162b2 vaccines. An der Goldgrube 12, Mainz 55131, Germany. © 2024 The Author(s). Published with license by Taylor & Francis Group, LLC. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. The terms on which this article has been published allow the posting of the Accepted Manuscript in a repository by the author(s) or with their consent. 2 F. VAN DEN OUWELAND ET AL. Figure 1. Accelerated timelines for the development and approval of COVID-19 vaccines.1,2,4 COVID-19, coronavirus disease 2019. Methods A literature search was performed in Medline (PubMed) and pre-print servers medRxiv and bioRxiv for English-language articles. No additional inclusion or exclusion criteria were applied. Additional references were obtained by searching citation lists of retrieved articles, and the authors identified further appropriate references for inclusion based on expert knowledge. Other sources of information included pharma­ ceutical company press releases and public health websites. Systematic search methodology was not used. The initial formal literature search, comprising terms such as BNT162b2, SARS-CoV-2, adverse event (AE), AE following immunization, and AE of special interest (AESI; including itemized terms such as ‘myocarditis’, ‘Bell’s Palsy’, ‘syncope’, etc.), was performed on October 05, 2023. After the date of the initial search, owing to the fast-moving nature of the field, additional targeted follow-up searches were performed throughout manuscript development, from first draft to finalization. Results Clinical development of BNT162b2 vaccines Clinical development of BNT162 vaccines was carried out in line with regulatory agency standards and applicable guidelines.5,8,9 Although timelines were compressed because of the public health emergency, the overall clinical develop­ ment process used was the same as that used for the develop­ ment and approval of other vaccines in terms of trials performed and data collected (Figure 1).10 Expectations for safety evaluations, including the volume and quality of data collected, were the same for vaccines developed in accelerated and non-emergency pre-approval environments.7 Both the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) devoted extra resources to support the rapid development and author­ ization of vaccines.7 The FDA EUA pathway enabled the pub­ lic to access COVID-19 vaccines in the emergency pandemic setting quickly while requiring manufacturers to supply addi­ tional vaccine data from clinical settings for rigorous review.7 Similarly, the EMA expedited its evidence appraisal process through rolling review stages,3,11 which allowed regulators to receive and review the data as soon as they became available. Regular dialogue between manufacturers, regulatory authori­ ties, and other stakeholders was essential in expediting time­ lines while adhering to the development process. Increased familiarity with the data, and preplanned opportunities for sponsor/regulator discussions, enabled regulators to provide expedited assessments of marketing authorization applications.3 Conditional marketing authorization for BNT162b2 was first granted by the European Commission on December 21, 2020.12 Vaccine development was also able to be expedited by building upon experience gained from development of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) vaccines,13 and because developers had clinical, regulatory, and manufacturing experience with mRNA formats and lipid nanoparticle (LNP)-based formulations.14,15 In accordance with guidance issued in early 2020, led by the International Coalition of Medicines Regulatory Authorities (ICMRA), wellcharacterized toxicology data could be leveraged across closely related vaccines using the same platform technology (e.g., same RNA formats and LNPs).16 Therefore, a platform-based toxicology program could be conducted to explore diverse combinations of mRNA chemistries and formats with different receptor-binding domain (RBD) or full-length SARS-CoV-2 HUMAN VACCINES & IMMUNOTHERAPEUTICS spike antigen designs in conjunction with the selected LNP formulation. Highly reproducible preclinical data were obtained from BNT162 vaccine candidates across independent toxicological evaluations in animal models.17 These efforts established an early platform toxicology profile that enabled rapid initiation of a first-in-human clinical trial investigating several of these vaccine candidates (Figure 1).17 Early stage clinical trials used sentinel cohorts and dose escalation to optimize enrollment rate and monitor safety, with stopping rules in place in the event of safety issues.18 An independent data monitoring committee reviewed safety data from clinical trials to deter­ mine whether any changes to planned doses should be made, and any treatment group should be terminated early.18 Double-blind study designs allowed for true assessment of safety events, and the large numbers of subjects enabled robust assessment of AEs. For example, clinical evaluation of BNT162b2 vaccines in approximately 20,000 vaccinated parti­ cipants enabled the detection of events with an incidence of > 1 event in 1,000 vaccinated individuals.19 Key safety and reacto­ genicity data from BNT162 preclinical and clinical trials are described below. Preclinical studies of original BNT162 candidate vaccines Preclinical assessment of BNT162 candidate vaccines was per­ formed in rats, mice, and monkeys. BNT162 vaccine candi­ dates coding for the RBD of the spike glycoprotein (S protein) have vaccine identifier names that end in “−1, −3,” whereas those coding for the full-length S protein have vaccine identi­ fier names that end in “−2” (Table 1).15 The preceding letter refers to distinct RNA platforms differing in chemistry or format used to construct the vaccine (a = unmodified uridinecontaining RNA [uRNA]; b = pseudouridine-modified RNA [modRNA]; c = self-amplifying RNA [saRNA]).15,20 All var­ iants were formulated with the same lipid formulation. 3 Five vaccine candidates were evaluated in preclinical repeat-dose toxicity studies in Wistar Han rats: four encoded different sequence variants on the RBD of the S protein (BNT162a1, BNT162b1, BNT162b3 and BNT162c1), and one encoded the full-length S protein in its pre-fusion conforma­ tion (BNT162b2, with sequence variants V8 and V9; Table 1A).17 Each candidate vaccine was tolerated without evidence of systemic toxicity at all doses evaluated, and vac­ cine-related findings for all candidates were similar. Clinical signs, including swelling at the injection site and increases in white blood cells, were as expected and consistent with vaccine administration and immune response; all effects were reversible.17 There were no effects of BNT162b2 on fertility parameters or fetal development.21,22 The BNT162b1 and BNT162b2 vaccine candidates were also evaluated in mice and rhesus macaques (Table 1A).23 Early clinical trials of original BNT162 vaccines Clinical dose-evaluation studies were performed to select the vaccine candidate with an optimal safety, reactogenicity, and immunogenicity profile.24,25 In an initial Phase I/II dose-escalation study conducted in Germany in adults 18–85 years of age (NCT04380701), four BNT162 vaccine candidates were evaluated: BNT162a1, BNT162b1, BNT162b2, and BNT162c2 (Table 1).26,27 Injection-site reactions within 7 days of administration were mainly injection-site pain and tenderness, and the severity of reported reactions was mostly mild or moderate, with an occasional severe (Grade 3) event. The incidence of reacto­ genicity events was dose-dependent. All AEs resolved sponta­ neously. No serious AEs were reported, and no withdrawals due to AEs were observed for any dose.27,28 A Phase I, randomized, placebo-controlled, dose-escalation trial in the United States in adults 18–55 years and 65–85 years of age evaluated BNT162b1 and BNT162b2 (NCT04368728).29 Table 1. BNT162 vaccine candidates evaluated in (A) preclinical trials and (B) early clinical trials. BNT162 vaccine candidate (product code)† Platform (A) Preclinical trials17,23 BNT162a1 uRNA BNT162b1 modRNA BNT162b2 modRNA Wild type Wild type Wild type BNT162b3 BNT162c1 Wild type Wild type modRNA saRNA SARS-CoV-2 variant† (B) Early clinical trials26–30,32,105 BNT162a1 uRNA Wild type BNT162b1 modRNA Wild type † Encoded antigen Evaluation SARS-CoV-2 RBD, a secreted variant SARS-CoV-2 RBD, a secreted variant Full-length SARS-CoV-2 S protein bearing mutations preserving neutralization-sensitive sites,§ a secreted variant SARS-CoV-2 RBD, a membrane-bound variant SARS-CoV-2 RBD, a secreted variant Rats‡ Rats, mice, rhesus macaques Rats, mice, rhesus macaques SARS-CoV-2 RBD, a secreted variant SARS-CoV-2 RBD, a secreted variant Germany, NCT04380701 Germany, USA, China NCT04380701 NCT04368728 NCT04523571 Germany, USA, Brazil, Argentina, Turkey, Germany NCT04380701 NCT04368728 Germany NCT04380701 BNT162b2 modRNA Wild type Full-length SARS-CoV-2 S protein bearing mutations preserving neutralization-sensitive sites, a secreted variant BNT162c2 saRNA Wild type Full-length SARS-CoV-2 S protein, a secreted variant Rats‡ Rats‡ Wild type refers to the USA-WA1/2020 strain. ‡Data on file. §Two sequence variants, V8 and V9, were evaluated in preclinical studies; V9 was included in the final approved vaccine. modRNA, modified RNA; RBD, Receptor-Binding Domain; S protein, spike protein; saRNA, self-amplifying RNA; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; uRNA, uridine RNA. 4 F. VAN DEN OUWELAND ET AL. The most common local reaction within 7 days after receipt of BNT162b1 and BNT162b2 was injection-site pain; this was more frequent after the second dose.29,30 Most local reactions and systemic events resolved by day 7.29,30 No participant who received either candidate reported a Grade 4 reactogenicity event, and there were no serious AEs.29 Systemic reactogeni­ city events were milder with BNT162b2 than with BNT162b1, and fewer recipients of BNT162b2 reported using antipyretic or pain medication, while the neutralizing antibody profiles of the vaccines were similar. BNT162b2, which encodes the fulllength SARS-CoV-2 S protein, was selected for further devel­ opment. The 30 μg dose was selected as the preferred dose level,29 driven by neutralizing antibody response and tolerabil­ ity data across all subgroups by age and sex. Similar safety and reactogenicity profiles were also observed for BNT162b1 in Chinese adults and for BNT162b2 in Japanese adults in Phase I and Phase I/II clinical trials (NCT04523571 and NCT04588480, respectively).31,32 Phase II/III clinical trials of original BNT162b2 The pivotal Phase II/III clinical trial of BNT162b2 (NCT04368728) was a continuation of the Phase I trial in the United States, expanded to a global scope. This trial rando­ mized 43,548 participants ≥16 years of age to receive two doses of 30 μg BNT162b2 or placebo, and included participants from 130 sites in the United States, Argentina, Brazil, South Africa, Germany, and Turkey.19 The overall safety population included 18,860 participants who received BNT162b2. In total, 58% were 16–55 years of age and 42% were >55 years of age.19 The prescribing information for BNT162b2 presents early reactivity data from the trial among 5,807 participants 16–55 years of age (2,682 randomized to BNT162b2 and 2,684 randomized to placebo; data cutoff March 13, 2021) (Table 2).33 In this dataset, the majority of local reactions after dose 2 were mild, with a mean duration of <3 days.33 In a larger reactogenicity subset (N = 8,183), the most commonly reported local reaction was injection-site pain within 7 days of dose 1 administration, occurring in 83% and 71% of BNT162b2-vaccinated participants 16–55 years of age and >55 years of age, respectively. The proportion of participants vaccinated with BNT162b2 reporting local reactions within 7 days of administration was lower after the second dose (78% and 66%, respectively).19 No participant reported a Grade 4 local reaction. Systemic events were more frequent after the second dose and in younger (16–55 years of age) versus older (≥55 years of age) participants.19 In total, ≤2% of participants vaccinated with BNT162b2 reported solicited systemic events graded as severe within 7 days of administration, with the exception of fatigue, which occurred in 3.8% of participants after dose 2.19 Two participants vaccinated with BNT162b2 reported a temperature of ≥40°C within 7 days of administration, as did two participants in the placebo group.19 Overall, reacto­ genicity events were transient and resolved within 1–2 days after onset.19 The reactogenicity profile of a larger subgroup of participants, including the initial 8,183 participants plus another 1,656 enrolled after the initial data cutoff, remained consistent with the earlier datasets.34 Among 43,252 participants with variable follow-up time for AEs, lymphadenopathy was reported by 64 (0.3%) BNT162b2 recipients and six (<0.1%) placebo recipients. This generally resolved within 10 days and was likely a result of a robust vaccine-elicited immune response in the BNT162b2 recipients.19 Participants were followed for serious AEs up to 6 months after dose 2. Four serious AEs considered to be related to vaccination were reported by recipients of Table 2. Frequency and percentages of participants with solicited local reactions, by maximum severity, within 7 days after each dose – participants 16–55 years of age – reactogenicity subset.†,33. Redness‖ Any (>2.0 cm) Mild Moderate Severe BNT162b2 Dose 1 N‡ = 2,899 n§ (%) Placebo Dose 1 N‡ = 2,908 n§ (%) BNT162b2 Dose 2 N‡ = 2,682 n§ (%) Placebo Dose 2 N‡ = 2,684 n§ (%) 156 (5.4) 113 (3.9) 36 (1.2) 7 (0.2) 28 (1.0) 19 (0.7) 6 (0.2) 3 (0.1) 151 (5.6) 90 (3.4) 50 (1.9) 11 (0.4) 18 (0.7) 12 (0.4) 6 (0.2) 0 16 (0.6) 6 (0.2) 8 (0.3) 2 (0.1) 183 (6.8) 110 (4.1) 66 (2.5) 7 (0.3) 5 (0.2) 3 (0.1) 2 (0.1) 0 414 (14.2) 391 (13.4) 20 (0.7) 3 (0.1) 2,101 (78.3) 1,274 (47.5) 788 (29.4) 39 (1.5) 312 (11.6) 284 (10.6) 28 (1.0) 0 Swelling‖ Any (>2.0 cm) 184 (6.3) Mild 124 (4.3) Moderate 54 (1.9) Severe 6 (0.2) Pain at the injection site¶ Any 2,426 (83.7) Mild 1,464 (50.5) Moderate 923 (31.8) Severe 39 (1.3) Reactions were collected in the electronic diary (e-diary) from day 1 to day 7 after vaccination. No Grade 4 solicited local reactions were reported in participants 16–55 years of age. †Randomized participants in the safety analysis population who received at least one dose of the study intervention. Participants with chronic, stable human immunodeficiency virus (HIV) infection were excluded. ‡N = number of participants reporting at least one yes or no response for the specified reaction after the specified dose. The N for each reaction was the same; therefore, this information was included in the column header. §N = number of participants with the specified reaction. ‖Mild: > 2.0 to ≤ 5.0 cm; moderate: > 5.0 to ≤ 10.0 cm; severe: > 10.0 cm. ¶Mild: does not interfere with activity; moderate: interferes with activity; severe: prevents daily activity. HUMAN VACCINES & IMMUNOTHERAPEUTICS BNT162b2; these were shoulder injury, right axillary lympha­ denopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia. No deaths were considered to be related to the vaccine, and few patients had AEs that led to trial withdrawal.19,34 After authorization of BNT162b2, participants were given the option to learn their trial assignment, and those receiving placebo were offered BNT162b2 in the context of the pandemic.34 To ensure long-term followup of both BNT162b2 and placebo recipients, participants continued to be followed after unblinding. No safety sig­ nals were observed during the extended follow-up period.34 BNT162b2 has also been evaluated in a Phase II trial in 960 Chinese adults 18–85 years of age, in which 720 indi­ viduals received BNT162b2 (NCT04649021). The reacto­ genicity and safety profile of BNT162b2 in this population was consistent with that of the global Phase II/III trial.35 Pediatric development Following authorization of the BNT162b2 vaccine for adult populations, a clinical trial was performed in participants 6 months–11 years of age to assess pediatric age-related doses of BNT162b2. In the Phase I portion, the cohort of children 5–11 years of age received two doses of BNT162b2 10 μg, 20 μg, or 30 μg (NCT04816643). Owing to a higher frequency of fever with the higher doses, and because the neutralizing antibody profiles of the 10 μg and 20 μg doses were similar, the 10 μg dose was selected for further assessment in the Phase II/III portion.36 In the cohort of children 6 months–4 years of age, participants received either 10 μg or 3 μg BNT162b2. Owing to a higher frequency and greater severity of reactogenicity to the 10 μg dose versus the 3 μg dose, and similar neutralizing antibody profiles across dose levels to that observed in older age groups, the 3 μg dose was selected for further assessment.37 The pivotal Phase II/III clinical trial of BNT162b2 (NCT04368728) also included a cohort of 2,260 participants 12–15 years of age, of whom 1,131 received BNT162b2. Solicited local and systemic events were generally mild or moderate in severity and were reported at a similar frequency to participants 16–25 years of age. One participant discontin­ ued the study due to a vaccine-related event of temperature ≥ 40°C after dose 1. Lymphadenopathy was reported by nine (0.8%) recipients of BNT162b2. Up to 1 month after dose 2, no vaccine-related serious AEs and no deaths had been reported.38 Of the 1,131 participants who received BNT162b2, 786 were followed for ≥4 months after the second dose, with the overall safety profile remaining similar to that seen in participants ≥16 years of age.21 In the Phase II/III portion of the clinical trial in children <12 years of age (NCT04816643), 1,517 children 5–11 years of age were randomized to receive 10 μg BNT162b2. Local reac­ tions were generally mild to moderate in intensity and lasted 1–2 days. Consistent with the trial in adults, injection-site pain was the most common local reaction, and fatigue and head­ ache were the most common solicited systemic reactions. One 5 recipient of BNT162b2 reported a temperature of ≥ 40°C after the second dose, which resolved with antipyretics.36 After a median follow-up time of 2.3 months after the second dose (95% of BNT162b2 recipients had a follow-up of ≥2 months), no vaccine-related serious AEs, AEs leading to withdrawal, or deaths had been reported.36 Safety evaluation in this study is ongoing.21 In a cohort of 3,013 children 6 months–4 years of age who received BNT162b2, most local and systemic reactions were mild to moderate in intensity and no Grade 4 local reactions were reported. The frequency of AEs was similar in BNT162b2 and placebo recipients, few participants were withdrawn due to AEs, and no deaths occurred.37 Booster doses The safety and reactogenicity of third and fourth doses of BNT162b2 were assessed in participants from the pivotal Phase II/III clinical trials. In 5,081 participants who received a third dose of BNT162b2, reactogenicity was similar to that observed after the second dose. With a median of 2.5 months of follow-up from dose 3, the safety profile of the vaccine was consistent with earlier trials, and no new safety signals were identified. Three participants who received BNT162b2 experi­ enced serious AEs that were considered to be related to the vaccine; these were tachycardia in one participant and increased hepatic enzyme levels in two participants.39 A subset of 306 participants 18–55 years of age were followed for a median of 8.3 months post-booster dose, with 301 fol­ lowed for ≥6 months. The overall safety profile of the booster dose remained consistent with that seen after two doses.21 In the trial in children <12 years of age, 401 participants 5– 11 years of age received a third dose at least 5 months (range 5– 9 months) after completing the primary series. With a median follow-up time of 1.3 months, the overall safety profile was similar to that seen after the primary course.21 In 570 infants 6–23 months of age with a median of 1.3 months of follow-up after dose 3, and 886 children 2–4 years of age with 1.4 months of follow-up after dose 3, reactogenicity after dose 3 was con­ sistent with the known safety profile of BNT162b2.21 In adults 18–55 years of age (N = 325) and >55 years of age (N = 305) who received a fourth dose of BNT162b2, the safety profile was similar to that observed after dose 3, after a median of 1.4 and 1.7 months follow-up post-dose 4, respectively.21 Because these studies enrolled participants from the previous trials who consented to further study, results may be subject to selection bias. Overall, the clinical development program for BNT162b2 included the largest pivotal registrational COVID-19 vaccine trial conducted to date and evaluated more than 44,000 parti­ cipants >12 years of age from around the world (Table 3).19,38 These findings were supported by further study in different world regions31,35 and Phase II/III trials in children >6 months of age.21,36,37 Variant-adapted vaccines The emergence of SARS-CoV-2 variants became apparent shortly after the first COVID-19 vaccines were deployed, Finland, Poland, Spain, United States Brazil, Finland, Poland, Spain, United States II/III United States II/III NCT04816643 I/II II/III NCT04368728 I Recipients (N,† age) N = 16 5–11 years 31.3% male, 68.8% White, 18.8% Black or African American, 12.5% Asian N = 16 6 months to <2 years 62.5% male, 87.5% White, 6.3% Asian N = 16 2–4 years 56.3% male, 75.0% White, 6.3% Asian N = 1,518 5–11 years 52.6% male, 79.3% White, 5.9% Black, 5.9% Asian N = 1,178 6 months to <2 years 50.0% male, 78.3% White, 3.6% Black, 7.7% Asian N = 1,835 2–4 years 49.1% male, 80.1% White, 5.1% Black, 6.9% Asian N = 12 18–55 years 66.7% male, 100% Caucasian United States N = 12 18–55 years 50% male, 83% White, 17% Asian N = 12 65–85 years 33% male, 83% White, 17% Asian Argentina, Brazil, N = 21720 Germany, South Africa, ≥16 years Turkey, United States 51.1% male, 82.9% White, 9.2% Black or African American, 4.2% Asian N = 1,131 12–15 years 50.1% male, 85.9% White, 4.6% Black or African American, 6.4% Asian Trial Phase Location Original vaccine primary series NCT04380701 I/II Germany Publication Two doses 3 μg Two doses 10 μg Two doses 3 μg Two doses 10 μg ● Most reactogenicity events were mild to moderate, no Grade 4 local reactions ● Few participants withdrawn due to AEs ● No deaths occurred follow-up of 2.3 months after dose 2 (95% with ≥2 months follow-up) (Continued) Munoz et al.37 ● Local and systemic reactions generally mild to moderate Walter et al.36 ● Severe reactions: injection-site pain 0.6%, fatigue 0.9%, headache 0.3% ● No vaccine-related serious AEs, AEs leading to withdrawal, or deaths with median ● Local and systemic reactions generally mild to moderate Munoz et al.37 Local and systemic reactions generally mild to moderate, resolved within 1–2 days Frenck et al.38 Severe injection-site pain in 1.5%, one discontinuation due to temperature ≥40°C EMA SmPC21 Lymphadenopathy in 0.8% No serious AEs related to vaccine In 786 participants with ≥4 months follow-up after dose 2, the safety profile remained similar to that in ≥16 years of age after ● Most local reactions mild to moderate, all transient Walter et al.36 Two doses 30 μg ● ● ● ● ● ● No Grade 4 local reactions; most local events resolved within 1–2 days Polack et al.19 ● Severe systemic events in ≤2% except fatigue (3.8%) within 7 days after dose 2 Thomas et al.34 ● Four related serious AEs and no vaccine-related deaths with up to 6 months follow-up Local reactions mostly mild to moderate in intensity, occasionally severe (Grade 3) Sahin et al.28 All AEs resolved spontaneously and were managed with simple measures No serious AEs or withdrawals due to related AEs Most common local reaction was injection-site pain, more frequent after dose 2 Walsh et al.29 No Grade 4 reactogenicity events Related AEs reported by 25% of participants 18–55 years of age and 0% participants 65–85 years of age ● No serious AEs ● ● ● ● ● ● Reactogenicity/safety Two doses 30 μg Two doses 30 μg Two doses 30 μg Doses Table 3. Safety data for approved BNT162b2 doses from clinical trials. Data for approved formulation and doses included only. 6 F. VAN DEN OUWELAND ET AL. China NCT04649021 II Location N = 97 20–64 years 51.5% male, 100% Asian N = 22 65–85 years 40.9% male, 100% Asian N = 720 18–85 years 51.5% male, 100% Chinese (Han ethnicity) Recipients (N,† age) N = 412 ≥12 years 41.3% male, 79.1% White, 12.6% Black, 5.3% Asian N = 74 ≥18 years N = 305 >55 years 53.1% male, 89.8% White, 4.3% Black, 5.2% Asian N = 305 >55 years N = 325 18–55 years N = 401 5–11 years N = 1,456 6 months to 4 years Doses Fourth to seventh dose 30 μg Fourth dose 30 μg Fourth dose 30 μg Fourth dose 30 μg Fourth dose 30 μg Third dose 10 μg Third dose 3 μg (any primary course dose) Third dose 30 μg Two doses 30 μg Two doses 30 μg † Total N randomized to BNT162b2 at dose shown. AE, adverse event; EMA, European Medicines Agency; SmPC, Summary of Product Characteristics. XBB.1.5 variant-adapted vaccine NCT05997290 II/III United States Original/Omicron BA.4–5 variant-adapted vaccine NCT05472038 II/III United States Original/Omicron BA.1 variant-adapted vaccine NCT04955626 III United States Not stated Not stated Not stated III NCT04816643 II/III Not stated III Original vaccine booster doses NCT04955626 II/III Brazil, South Africa, United N = 5,081 States ≥16 years 48.4% male, 78.7% White, 9.3% Black, 5.7% Asian Japan Phase NCT04588480 I/II Trial Table 3. (Continued). Most reactogenicity events mild to moderate No Grade 4 reactogenicity events No new adverse reactions identified No vaccine-related serious AEs, AEs leading to withdrawal, or deaths ● ● ● ● After 1 month follow-up, most reactogenicity events mild to moderate AEs infrequent (7.5% in the total population); none led to study withdrawal No myocarditis or pericarditis reports No new safety signals ● Safety profile similar to that of the original vaccine ● ● ● ● children 2–4 years of age with 1.4 months follow-up after dose 3, reactogenicity after dose 3 was consistent with the known safety profile of BNT162b2 Gayed et al.42 BioNTech41 Winokur et al.40 ● In 570 infants 6–23 months of age with median 1.3 months follow-up, and 886 EMA SmPC21 similar to that seen after the primary course ● With median follow-up time of 1.3 months after dose 3, overall safety profile was EMA SmPC21 known safety profile of BNT162b2 ● With median follow-up of 1.4 months after dose 4, reactogenicity was consistent with EMA SmPC21 seen after dose 3 ● With median follow-up of 1.7 months after dose 4, the safety profile was similar to that EMA SmPC21 identified ● Three vaccine-related serious AEs ● Subset of 306 participants followed for median 8.3 months, no new safety signals consistent with primary series ● Reactogenicity after dose 3 similar to that after dose 2 Moreira et al.39 ● With median follow-up of 2.5 months after dose 3, the safety profile remained EMA SmPC21 Hui et al.35 ● Most reactogenicity events mild to moderate and transient ● No vaccine-related serious AEs or deaths Publication Haranaka et al.31 Reactogenicity/safety ● Most common local reaction: injection-site pain ● Reactogenicity events mild to moderate and generally transient ● No serious/life-threatening AEs and no deaths HUMAN VACCINES & IMMUNOTHERAPEUTICS 7 8 F. VAN DEN OUWELAND ET AL. Table 4. Surveillance systems. Passive surveillance systems Purpose Collect spontaneous AE reports Example CDC VAERS in the United States and the EMA EudraVigilance system in the European Union57,61,62 Active surveillance systems Collect all reports of pre-specified AEs from a representative population, such as a sentinel site United States VSD and BEST61,62 Other Track post-marketing surveillance AEs Collaborate with academic and nongovernment healthcare systems CISA and the CBER61,62 WHO VigiBase captures reports from national centers from over 130 countries63 AE, Adverse Event; BEST, Biologics Effectiveness and Safety; CBER, Center for Biologics Evaluation and Research; CDC, United States Centers for Disease Control and Prevention; CISA, CDC Clinical Immunization Safety Assessment; EMA, European Medicines Agency; VAERS, Vaccine Adverse Event Reporting System; VSD, Vaccine Safety Datalink; WHO, World Health Organization. prompting developers to evaluate updated vaccine candidates. When the Omicron BA.1 variant of SARS-CoV-2 and its sub­ sequent lineages emerged, BioNTech/Pfizer pursued the devel­ opment of several variant-adapted vaccines, including a monovalent BA.1 vaccine candidate, a bivalent vaccine can­ didate targeting both the original wild-type virus and BA.1, and a bivalent vaccine targeting the original wild-type virus and BA.4/BA.5.40 In view of the public health urgency for protection against Omicron subvariants, the bivalent vaccines encoding the Original/Omicron BA.1 and Original/Omicron BA.4/5 spike proteins were approved by regulators in different regions. These approvals were based on clinical trials con­ ducted with BA.1 vaccine candidates (monovalent and biva­ lent, at different dose levels) demonstrating significant increases in neutralizing antibody titers,40 as well as precli­ nical immunogenicity data, and were supported by the extensive and robust safety database for the original BNT162b2 vaccine and the clinical data generated across multiple variant-adapted vaccine candidates encoding earlier SARS-CoV-2 variants.43 In parallel, clinical trials were initiated to further evaluate the safety and reactogenicity of these vaccines. In a Phase III clinical trial, adults >55 years of age who had previously received three doses of the original BNT162b2 vaccine were randomized to receive either bivalent Original/ Omicron BA.1 BNT162b2 vaccine or original BNT162b2 (NCT04955626) (Table 3). In total, 306 participants were ran­ domized to receive a 30 μg dose and 316 received a 60 μg dose of bivalent vaccine.40 The bivalent Original/Omicron BA.1 BNT162b2 vaccine had a similar local reaction and systemic event profile to the original BNT162b2 vaccine.21,40 Most reactogenicity events were mild to moderate in intensity and no Grade 4 reactogenicity events were reported. Injection-site pain and fatigue were the most common local and systemic reactogenicity events, respectively. In participants >55 years of age, mild-to-moderate injection-site pain, fatigue, and muscle pain were more common with the 60 μg dose than with the 30 μg dose of bivalent vaccine.44 No serious AEs were considered related to the bivalent vaccine, and there were no AEs leading to withdrawal or deaths.40 No new safety signals were detected.21 The 30 μg bivalent Original/Omicron BA.4–5 vaccine was assessed in a Phase II/III trial in adults 18–55 years of age and >55 years of age who had previously received three doses of the original BNT162b2 vaccine (NCT05472038).45 Early clinical safety data from 7 and 30 days post-vaccination indicate that the bivalent Original/Omicron BA.4–5 BNT162b2 vaccine is well tolerated, with a safety profile similar to that of the original vaccine.41,45 Continued evolution of SARS-CoV-2 led to recommenda­ tions from the WHO Technical Advisory Group on COVID19 Vaccine Composition (TAG-CO-VAC) to include a component of an XBB.1 descendant sub-lineage of SARSCoV-2 in COVID-19 vaccines for fall 2023.46 Following con­ sistent guidance issued by the EMA/European Centre for Disease Control and Prevention,47 and by the FDA,48 BioNTech and Pfizer developed a monovalent XBB.1.5 vaccine that has received regulatory approvals in various countries.49,50 1-month safety follow-up from an ongoing Phase II/III study of this vaccine in participants ≥12 years of age (NCT05997290) did not detect any new safety signals. Local reactions and systemic events were mostly mild to moderate in severity, AEs were infrequent (7.5% of the total population), and no AEs led to study withdrawal.42 Post-marketing surveillance and real-world data In both the United States and European Union, postauthorization safety monitoring and risk minimization proce­ dures were put in place following BNT162b2 commercializa­ tion. International Risk Management Plans (RMPs), or comprehensive documents summarizing the vaccine safety profile and measures taken to further investigate and mitigate risks, were submitted alongside dossiers for marketing authorization.51 Post-authorization commitments included in the European Union RMP included studies to assess the risk of vaccine-associated enhanced disease, impacts on special popu­ lations (including pregnant/breastfeeding women and those with compromised immune systems or comorbidities), and the potential for interactions between vaccines, as well as studies to obtain longer-term safety data.52 After FDA approval following a period of EUA, additional postmarketing studies to assess the risk of myocarditis and peri­ carditis were required. A registry to evaluate pregnancy and infant outcomes was also established.53 In the European Union, the EMA Pharmacovigilance Risk Assessment Committee published regular safety update reports based on data from the marketing authorization holder and data reported by either patients or healthcare providers to EudraVigilance.12,54 Post-marketing safety reporting for COVID-19 vaccines has been extremely robust compared with other vaccines. COVID-19 vaccine safety surveillance in the United States has been described as the most intensive in the country’s history.55 During the peak of the pandemic, manufacturers of HUMAN VACCINES & IMMUNOTHERAPEUTICS COVID-19 vaccines were required to submit monthly safety reports to the EMA.56 These safety updates subsequently reduced in frequency and were eventually discontinued when the EMA concluded that the safety profile of the vaccines had been well established.56,57 Monthly safety reports continue to be submitted to the FDA.58 Marketing authorization holders are legally obliged to set up and maintain database systems for authorized products to receive, record, and analyze safety data and signals from spontaneous reporting.59 Post-authorization, the safety of BNT162b2 and other COVID-19 vaccines has been monitored through both passive and active surveillance systems.60 Together, these surveillance systems (Table 4) capture safety data from an extensive and heterogenous global population. In European Union/ European Economic Area countries alone, as of June 16, 2023, almost 535 million doses of original BNT162b2 and 32 million doses of bivalent BNT162b2 have been administered.64 In the United States, as of May 10, 2023, more than 360 million and 36 million doses, respectively, have been administered.65 When rare events are detected by a safety monitoring sys­ tem, manufacturers, as well as regulatory bodies such as the FDA and EMA, review the signals. This is done, for example, by comparing rates in vaccinated and unvaccinated people, comparing rates in an early reporting period with a later inter­ val when more data have been collected, and comparing with data from other surveillance systems, databases, and published literature. If an event is determined as likely to be associated with vaccination, regulatory agencies may then request changes to vaccine recommendations or product information. Monitoring and analysis of the event will continue in order to detect any changes to a signal, until the event is closed.55,57 Implementation of mass vaccination programs also enables real-world studies of vaccine safety in vaccinated populations. These studies can complement post-marketing surveillance by providing additional data on safety signals. They may also provide estimates on more common events, such as reacto­ genicity, in different populations from those enrolled in the clinical trials. For COVID-19 vaccines, real-world studies had an important impact on post-authorization regulatory agency and government decision-making due to their unprecedented number, early conduct, and rapid publication. Selected AESIs reported with BNT162b2 vaccination AESIs are pre-identified and pre-defined serious or nonserious AEs of scientific and medical interest for which ongoing monitoring, further investigation, and/or rapid com­ munication by the sponsor to the regulator or other stake­ holders may be appropriate.66,67 AESIs are usually identified through active vaccine safety surveillance systems.67 Prior to vaccine use in the community, AESIs are identified based on known occurrence patterns in the population and their poten­ tial to be associated with one or more vaccine platforms.67 AE case definitions are developed by the Brighton Collaboration, which also maintains a list of vaccine platform-related AESIs.11,67,68 AESIs that have been reported following BNT162b2 vaccination include Bell’s palsy, and myocarditis and pericarditis. Facial paralysis and swelling, bell’s palsy Acute peripheral facial paralysis, or Bell’s palsy, is a rare event associated with BNT162b2 vaccination, occurring in ≥1 in 10,000 to <1 in 1,000 recipients (Table 5).21 This rare event was initially detected during clinical trials,21 and cases have since been observed post-BNT162b2 administration during mass vaccination campaigns,69,70 although a causal association between these events and BNT162b2 vaccination has not been clearly demonstrated.71,72 In case – control studies in Hong Kong and Israel, rates of facial nerve palsy/Bell’s palsy reported after COVID-19 vaccination were similar to back­ ground rates recorded during years prior to the emergence of COVID-19.71,73 Facial nerve palsy has been described as an AE following administration of other vaccines, including influenza, varicella, human papillomavirus, diphtheria – tetanus – pertussis, and meningococcal vaccines.74 Causality is not confirmed, with the exception of an intranasal influenza vaccine containing Escherichia coli heat-labile toxin as an adjuvant, leading to the hypothesis that this toxin or the route of administration may be a cause.72,75 BNT162b2 does not include this toxin and uses a different mechanism to stimulate an immune response.71 Myocarditis and pericarditis Myocarditis/pericarditis is a very rare AE that has been observed after administration of COVID-19 mRNA vaccines (Table 5).76 Owing to its rarity, this event was not detected in clinical trials, but was reported post-authorization.21 Myocarditis and pericarditis are considered important identi­ fied risks for COVID-19 mRNA vaccines, including original BNT162b2 vaccine and variant-adapted vaccines, and are included and described accordingly in the European Union RMP and aggregate safety reports. The frequency varies Table 5. Frequency of selected adverse events and AESIs following BNT162b2 vaccination in individuals ≥12 years of age based on clinical trials and post-authorization experience21. Adverse event/AESI Acute peripheral facial paralysis (Bell’s palsy) Myocarditis/pericarditis Hypersensitivity reactions Heavy menstrual bleeding Anaphylaxis† † 9 Frequency Rare (≥ 1/10,000 to < 1/1,000) Very rare (< 1/10,000) Uncommon (≥ 1/1,000 to < 1/100) Not known (cannot be estimated from available data) Not known (cannot be estimated from available data) Appropriate medical treatment and supervision should always be readily available in case of an anaphylactic reaction following administration of the vaccine. Close observation for at least 15 minutes is recommended following vaccination. No further dose of the vaccine should be given to those who have experienced anaphylaxis after a prior dose.21 AESI, adverse event of special interest. 10 F. VAN DEN OUWELAND ET AL. depending on the mRNA vaccine used.77 After BNT162b2 vaccination, myocarditis has occurred in <1 in 10,000 recipients.21 Myocarditis has been reported within a few days of vacci­ nation, usually within 14 days. It is more frequently observed after the second dose than the first dose of the vaccine, and its incidence is highest in young men.21 A large European phar­ macoepidemiologic study estimated that the excess risk of myocarditis over the 7 days post-dose 2 was 0.265 (95% con­ fidence interval [CI]: 0.255–0.275) extra cases per 10,000 for men 12–29 years of age, compared with unexposed persons. Another similar study estimated an excess of 0.56 extra cases per 10,000 during the 28 days post-dose 2 in men 16–24 years of age.21 In the United States, the estimated incidence of myocarditis during the 7 days post-dose 2 of any COVID-19 mRNA vaccine based on cases reported to the United States Centers for Disease Control and Prevention (CDC) Vaccine Adverse Event Reporting System (VAERS) was 40.6 cases per million doses in men 12–29 years of age and 2.4 per million in those ≥30 years of age; corresponding rates for women were 4.2 and 1.0 per million, respectively.76 Estimates from a systematic literature review reported a range of 50–139 and 28–147 cases per million in young men 12–17 years of age and 18–29 years of age, respectively, after COVID-19 mRNA vaccination.77 The clinical course and prognosis of post-vaccination myo­ carditis is comparable with myocarditis of other causes.54 In fact, the available literature supports that COVID-19 vaccineassociated myocarditis has a milder presentation, higher recov­ ery rate, and lower mortality compared with myocarditis of other causes (Table 6).78–82 In the few cases observed, the severity of myocarditis and pericarditis following BNT162b2 vaccination varied; most patients responded well to medications and rest, with prompt improvement of symptoms. Preliminary United States CDC surveys conducted ≥90 days post-diagnosis showed that most patients fully recovered.83 Data from the United States CDC, VAERS, and a European observational study suggest that SARS-CoV-2 infection-related myocarditis occurs in 15–400 people per 10,000 infected.84–87 Based on a study evaluating magnetic resonance imaging from the University of Toronto and a systematic literature review, the patterns of myocardial injury with vaccine‐associated myocar­ ditis are similar to those seen with idiopathic or viral myocardi­ tis, such as that induced by COVID-19.81,82 Immunopathology data from the United States suggest that myocarditis postvaccination might be driven by elevation of circulating inflam­ matory cytokines and corresponding lymphocytes, with no evi­ dence of cardiac-targeted autoantibodies, hypersensitivity, or hyperimmune humoral mechanisms.88 Other select AEs following BNT162b2 immunization Hypersensitivity Hypersensitivity to BNT162b2 has been reported, with symp­ toms such as rash and pruritus occurring in ≥1 in 1,000 to <1 in 100 recipients ≥12 years of age (Table 5).21 Allergic reac­ tions may be due to active ingredients or excipients used in the vaccine formulation.89 The incidence of anaphylaxis (severe, life-threatening allergic reaction) is low and, thus, difficult to estimate from available data.21 After administration of 1,893,360 first doses of BNT162b2, 21 cases meeting Brighton Collaboration cri­ teria for anaphylaxis were identified in the CDC’s VAERS, corresponding to a rate of 11.1 cases per million doses.90 A later report after 9,943,247 doses of BNT162b2 estimated a rate of 4.7 cases of anaphylaxis per million doses administered.91 In total, 77% of individuals had a past history of allergic reaction, and 34% had a past history of anaphylaxis.91 Although anaphylaxis was one of the first safety signals to be detected with BNT162b2 during post-marketing surveil­ lance, with progressive exposure, it became apparent that the cumulative number of cases was very small.12 Late hypersen­ sitivity reactions (appearing or lasting >24 h after vaccina­ tion) linked with prior exposure to hyaluronic acid dermatological fillers have been reported in a study in Israel, but these did not prevent individuals from receiving subsequent doses of the vaccine.92 Anxiety-related reactions Anxiety-related reactions, such as syncope, dizziness, palpita­ tions, increases in heart rate, alterations in blood pressure, paresthesia, hypoesthesia, and sweating, may occur after BNT162b2 vaccination. These are not AEs caused by the vaccine formulation, but are rather due to stress and anxiety associated with the vaccination process itself.21 These stressrelated reactions are temporary and will resolve on their own. Individuals suffering from anxiety should bring symptoms to the attention of the vaccination provider for evaluation. Precautions should be taken to avoid injury from fainting.21 Heavy menstrual bleeding Heavy menstrual bleeding (defined as increased volume or dura­ tion that interferes with quality of life) has been reported after BNT162b2 administration.21,93,94 The incidence of this event is unknown (Table 5), and based on evidence from nearly 9,000 cases from clinical trials, observational studies, post-marketing surveillance activities, and spontaneous reports, most cases have appeared to be non-serious and temporary in nature.94 A small number of cases involving positive re-challenge suggested a possible causal relationship.94 COVID-19 vaccina­ tion is a powerful immune stimulant; thus, it could be hypothesized that the sensitive immune system of the endo­ metrium is briefly modified by vaccination, potentially leading to menstrual disorders.95 However, other studies have failed to detect an association between change in menses cycle length and COVID-19 vaccination.96 Menstrual disorders can occur for a wide range of reasons, including some underlying med­ ical conditions.94 There is no evidence to suggest that men­ strual changes occurring after BNT162b2 vaccination have any impact on fertility.94 Comparison with other prophylactic vaccines An analysis of the WHO international database, VigiBase, comparing the incidence of AEs following COVID-19 mRNA vaccination with that of approved influenza vaccines, showed that serious cardiovascular events were more prevalent with Nationwide register data 7,292 patients with new onset myocarditis (530 associated with mRNA vaccination, 109 associated with COVID-19, 6,653 with conventional myocarditis), within a population of 23 million individuals Population 1,612 cases of myocarditis, within a population of 32 million 12–50 years of age (21.2 million received first and 19.3 million received second doses of BNT162b2; 2.86 million received first and 2.58 million received second doses of mRNA-1273) Self-controlled case series 2,861 cases of myocarditis, within a population of 42,842,345 people receiving at least one dose of COVID-19 vaccine Design Matched case – control study CI, confidence interval; COVID-19, coronavirus disease 2019; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Denmark, Finland, Norway, and Sweden Husby et al. 202380 Location England France Patone et al. 202279 Publication Le Vu et al. 202278 Table 6. Key studies comparing post-vaccination myocarditis with myocarditis from other causes including COVID-19. Outcomes ● ● ● ● tion, and death was observed in patients with post-mRNA vaccination myocarditis than in unvaccinated patients with myocarditis from any cause Risk of myocarditis was increased in the 1 to 28 days after a first, second, and booster dose of BNT162b2 (1.52 [95% CI 1.24–1.85], 1.57 [95% CI 1.28–1.92], and 1.72 [95% CI 1.33–2.22]) but was lower than the risks after a positive SARS-CoV-2 test before or after vaccination (11.14 [95% CI 8.64–14.36] and 5.97 [95% CI 4.54–7.87]) At 90 days follow-up, relative risk of heart failure was higher for myocarditis associated with COVID-19 than for myocarditis associated with vaccination (1.48 [95% CI 0.86–2.54] and 0.56 [95% CI 0.37–0.85] versus conventional myocarditis) At 90 days follow-up, relative risk of death was higher for myocarditis associated with COVID-19 than for myocarditis associated with vacci­ nation (2.35 [95% CI 1.06–5.19] and 0.48 [95% CI 0.21–1.09] versus conventional myocarditis) Among patients 12–39 years of age with no predisposing comorbidities, the relative risk of heart failure or death was markedly higher for myocarditis associated with COVID-19 disease than for myocarditis associated with vaccination (relative risk 5.78 [95% CI 1.84–18.20]) ● A lower frequency of intensive care unit admission, mechanical ventila­ HUMAN VACCINES & IMMUNOTHERAPEUTICS 11 12 F. VAN DEN OUWELAND ET AL. the COVID-19 vaccines, while influenza vaccines were more frequently associated with neurological AEs.97 Overall, the risk of serious events with the COVID-19 mRNA vaccines was lower than that of the influenza vaccines. Similarly, in a study evaluating AEs in children <5 years of age in Germany, symptoms following BNT162b2 vaccination were generally comparable to those following non-SARS-CoV-2 vaccines including influenza, meningococcal, measles – mumps – rubella, tetanus – diphtheria – pertussis, hepatitis A/B, and human papillomavirus vaccines.98 Discussion The overall experience accrued over time on the safety and reactogenicity of BNT162b2 is extensive, robust, and compre­ hensive, and it has confirmed the initial safety profile observed in clinical trials, demonstrating that the vaccine is well toler­ ated. Most adverse reactions are transient, can be managed with medications, or can be reduced through preventive mea­ sures. The BNT162 original and variant-adapted vaccines have been well tolerated, and their safety and reactogenicity profiles have remained consistent throughout the clinical development process, which included the largest pivotal registrational COVID-19 vaccine trial conducted to date. Occurrences of suspected AEs following vaccination should be interpreted in the context of overall numbers of doses administered and background rates of events. Serious AEs potentially related to vaccination are very rare21,99; therefore, vaccination should be encouraged, as it provides protection against a potentially deadly illness. Although the benefit – risk profile of BNT162b2 varies by age and sex, the prevention of hospitalization, severe disease, and death outweighs the risk of AEs after vaccination in the populations for which vaccination is recommended. Vaccine-associated myocarditis/pericarditis is a rare adverse reaction identified for COVID-19 vaccines. As the risk of myocarditis/pericarditis resulting from COVID-19 disease is higher than the risk of myocarditis/pericarditis asso­ ciated with vaccination,100 and as the benefits of prevented COVID-19 cases and related severe outcomes outweigh the risks of myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines, the overall benefit – risk profile of BNT162b2 is positive.83,101 It has been estimated that COVID-19 vaccines saved 19.8 million lives during the first year of rollout alone.102 Vaccine developers continue to collaborate with regulatory authorities for ongoing safety surveillance of COVID-19 vac­ cines as a key priority, while variant-adapted vaccines are rolled out.49,50,103,104 Ongoing collection of safety data for the XBB.1.5 monovalent vaccine, as well as any future variantadapted vaccines, will continue.105 In summary, the BNT162b2 vaccines have undergone thor­ ough and extensive safety testing and monitoring, consistently demonstrating a favorable safety profile. Although no longer considered a public health emergency, COVID-19 continues to pose a threat to public health. Vaccines are critical for disease prevention and have been an essential tool in the management of the COVID-19 pandemic. Annual immunization against SARS-CoV-2 may be an option for the future. Education for healthcare providers and the general public regarding the safety of BNT162b2 and other COVID-19 vaccines are essen­ tial to ensure the success of vaccination programs. Acknowledgments Medical writing support, including assisting authors with the develop­ ment of the outline and initial draft and incorporation of comments was provided by Rachel Wright, PhD, and Helene Wellington, MS, and editorial support was provided by Ian Norton, PhD, all of Scion, London, UK, supported by BioNTech SE according to Good Publication Practice guidelines (Link). Disclosure statement ÖT is a management board member and employee at BioNTech SE (Mainz, Germany) and co-founder of the company. FvO, NC, FJM, CL, SP, and RR are employees at BioNTech SE. ÖT is an inventor on patents and patent applications related to RNA technology and COVID-19 vac­ cines. SP, RR, CL and ÖT have securities from BioNTech SE. Funding The work was supported by the BioNTech . Author contributions All authors contributed to the manuscript conception, writing, and review process, and approved the final version for submission. References 1. Kassianos G, Puig-Barbera J, Dinse H, Teufel M, Tureci O, Pather S. Addressing COVID-19 vaccine hesitancy. Drugs Context. 2022;11: doi:10.7573/dic.2021-12-3. 2. Kalinke U, Barouch DH, Rizzi R, Lagkadinou E, Tureci O, Pather S, Neels P. Clinical development and approval of COVID-19 vaccines. Expert Rev Vaccines. 2022;21(5):609–19. doi:10.1080/14760584.2022.2042257. 3. Marinus R, Mofid S, Mpandzou M, Kühler TC. Rolling Reviews During COVID-19: The European Union Experience in a Global Context. Clin Ther. 2022;44:352–63. doi:10.1016/j.clinthera.2022.01.001. 4. Vaccine Research and Development. [Internet]. Johns Hopkins University & Medicine; 2023 [accessed 2023 Mar 3]. https://coro navirus.jhu.edu/vaccines/timeline. 5. Emergency use authorization for vaccines to prevent COVID-19: guidance for industry. [Internet]. US Food And Drug Administration; 2022 [accessed 2022 Jul 12]. https://www.fda. gov/media/142749/download. 6. Pfizer and BioNTech submit a variation to EMA with the data in support of a booster dose of COMIRNATY®. [Internet]. Pfizer; 2021 [accessed 2023 May 1]. https://www.pfizer.com/news/ announcements/pfizer-and-biontech-submit-variation-ema-datasupport-booster-dose-comirnatyr. 7. Cole A, Webster P, Van Liew D, Salas M, Aimer O, Malikova MA. Safety surveillance and challenges in accelerated COVID-19 vac­ cine development. Ther Adv Drug Saf. 2022;13:20420986221116452. doi:10.1177/20420986221116452. 8. EMA considerations on COVID-19 vaccine approval. [Internet]. European Medicines Agency; 2020 [accessed 2023 Feb 27]. https:// www.ema.europa.eu/en/documents/other/ema-considerationscovid-19-vaccine-approval_en.pdf. 9. Reflection paper on the regulatory requirements for vaccines intended to provide protection against variant strain(s) of SARS-CoV-2. [Internet]. European Medicines Agency; 2021 [accessed 2021 February 23]. https://www.ema.europa.eu/en/docu HUMAN VACCINES & IMMUNOTHERAPEUTICS 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. ments/scientific-guideline/reflection-paper-regulatory-requirementsvaccines-intended-provide-protection-against-variant_en.pdf. COVID-19 vaccines: development, evaluation, approval and mon­ itoring. [Internet]. European Medicines Agency; 2021 [accessed 2021 Jun 18]. https://www.ema.europa.eu/en/human-regulatory /overview/public-health-threats/coronavirus-disease-covid-19/ treatments-vaccines/vaccines-covid-19/covid-19-vaccinesdevelopment-evaluation-approval-monitoring. Durand J, Dogne JM, Cohet C, Browne K, Gordillo-Maranon M, Piccolo L, Zaccaria C, Genov G. Safety monitoring of COVID-19 vaccines: perspective from the European Medicines Agency. Clin Pharmacol Ther. 2023;113:1223–34. doi:10.1002/cpt.2828. Comirnaty. [Internet]. European Medicines Agency; 2023 [accessed 2023 Feb 23]. https://www.ema.europa.eu/en/medi cines/human/EPAR/comirnaty. Younus MM, Al-Jumaili AA. An overview of COVID-19 vaccine safety and post-marketing surveillance systems. Innov Pharm. 2021;12:10. doi:10.24926/iip.v12i4.4294. Jung HN, Lee SY, Lee S, Youn H, Im HJ. Lipid nanoparticles for delivery of RNA therapeutics: current status and the role of in vivo imaging. Theranostics. 2022;12:7509–31. doi:10.7150/thno.77259. Pascolo S. Vaccines against COVID-19: priority to mRNA-based formulations. Cells. 2021;10:2716. doi:10.3390/cells10102716. Global regulatory workshop on COVID-19 vaccine development. [Internet]. International coalition of medicines regulatory authorities; 2020 [accessed 2023 May 23]. https://www.icmra. info/drupal/news/March2020/summary. Rohde CM, Lindemann C, Giovanelli M, Sellers RS, Diekmann J, Choudhary S, Ramaiah L, Vogel AB, Chervona Y, Muik A. et al. Toxicological assessments of a pandemic COVID-19 vaccine— demonstrating the suitability of a platform approach for mRNA vaccines. Vaccines (Basel). 2023;11(2):417. doi:10.3390/vac cines11020417. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577. Investigator’s brochure, BNT162/PF-07302048. [Internet]. BioNtech; 2020 [accessed 2023 May 23]. https://www.tga.gov.au/ sites/default/files/foi-2183-09.pdf. Comirnaty COVID-19 vaccine. Summary of product characteris­ tics. [Internet]. European Medicines Agency; 2022 [accessed 2022 Sep 7]. https://www.ema.europa.eu/en/documents/productinformation/comirnaty-epar-product-information_en.pdf. Bowman CJ, Bouressam M, Campion SN, Cappon GD, Catlin NR, Cutler MW, Diekmann J, Rohde CM, Sellers RS, Lindemann C. Lack of effects on female fertility and prenatal and postnatal off­ spring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol. 2021;103:28–35. doi:10. 1016/j.reprotox.2021.05.007. Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, Kranz LM, Walzer KC, Hein S, Guler A. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–9. doi:10.1038/s41586-021-03275-y. Mahanty S, Prigent A, Garraud O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol. 2015;16:31. doi:10.1186/s12865-015-0095-y. Ashmawy R, Hamdy NA, Elhadi YAM, Alqutub ST, Esmail OF, Abdou MSM, Reyad OA, El-Ganainy SO, Gad BK, Nour El-Deen AE. et al. A meta-analysis on the safety and immunogenicity of covid-19 vaccines. J Prim Care Community Health. 2022;13:21501319221089255. doi:10.1177/21501319221089255. A trial investigating the safety and effects of four BNT162 vaccines against COVID-2019 in healthy and immunocompromised adults (NCT04380701). [Internet]. Clinicaltrials.gov; 2023 [accessed 2023 Feb 22]. https://clinicaltrials.gov/ct2/show/NCT04380701. 13 27. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–9. doi:10.1038/s41586-020-2814-7. 28. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, Quandt J, Bidmon N, Ulges A, Baum A. et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–7. doi:10. 1038/s41586-021-03653-6. 29. Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R. et al. Safety and immunogenicity of two RNA-Based covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi:10.1056/ NEJMoa2027906. 30. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA. et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–93. doi:10.1038/s41586-020-2639-4. 31. Haranaka M, Baber J, Ogama Y, Yamaji M, Aizawa M, Kogawara O, Scully I, Lagkadinou E, Tureci Ӧ, Sahin U. et al. A randomized study to evaluate safety and immunogenicity of the BNT162b2 COVID-19 vaccine in healthy Japanese adults. Nat Commun. 2021;12:7105. doi:10.1038/s41467-021-27316-2. 32. Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, Ji R, Lin M, Zhu Z, Tureci O. et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat Med. 2021;27(6):1062–70. doi:10.1038/s41591-021-01330-9. 33. COMIRNATY Package Insert [Internet]. Food and Drug Administration (FDA). 2023 [accessed 2023 May 23]. https:// www.fda.gov/media/151707/download. 34. Thomas SJ, Moreira ED Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Polack FP, Zerbini C. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–73. doi:10. 1056/NEJMoa2110345. 35. Hui AM, Li J, Zhu L, Tang R, Ye H, Lin M, Ge L, Wang X, Peng F, Wu Z. et al. Immunogenicity and safety of BNT162b2 mRNA vaccine in Chinese adults: a phase 2 randomised clinical trial. Lancet Reg Health West Pac. 2022;29:100586. doi:10.1016/j. lanwpc.2022.100586. 36. Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, Barnett ED, Munoz FM, Maldonado Y, Pahud BA. et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. doi:10.1056/ NEJMoa2116298. 37. Munoz FM, Sher LD, Sabharwal C, Gurtman A, Xu X, Kitchin N, Lockhart S, Riesenberg R, Sexter JM, Czajka H. et al. Evaluation of BNT162b2 COVID-19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388:621–34. doi:10.1056/ NEJMoa2211031. 38. Frenck RW Jr., Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R. et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vac­ cine in adolescents. N Engl J Med. 2021;385(3):239–50. doi:10. 1056/NEJMoa2107456. 39. Moreira ED Jr., Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, Perez JL, Zerbini C, Dever ME, Jennings TW. et al. Safety and efficacy of a third dose of BNT162b2 covid-19 vaccine. N Engl J Med. 2022;386:1910–21. doi:10.1056/NEJMoa2200674. 40. Winokur P, Gayed J, Fitz-Patrick D, Thomas SJ, Diya O, Lockhart S, Xu X, Zhang Y, Bangad V, Schwartz HI. et al. Bivalent omicron BA.1–adapted BNT162b2 booster in adults older than 55 years. N Engl J Med. 2023;388(3):214–27. doi:10. 1056/NEJMoa2213082. 41. Pfizer and BioNTech Announce Updated Clinical Data for Omicron BA.4/BA.5-Adapted Bivalent Booster Demonstrating Substantially Higher Immune Response in Adults Compared to the Original COVID-19 Vaccine. [Internet]. BioNtech; 2022 [accessed 2023 Mar 29]. https://www.pfizer.com/news/press- 14 F. VAN DEN OUWELAND ET AL. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. release/press-release-detail/pfizer-and-biontech-announceupdated-clinical-data-omicron. Gayed J, Diya O, Lowry FS, Xu X, Bangad V, Mensa F, Zou J, Xie X, Hu Y, Lu C. et al. Safety and immunogenicity of the monovalent omicron XBB.1.5-adapted BNT162b2 COVID-19 vaccine in indivi­ duals ≥12 years old: a phase 2/3 trial. Vaccines. 2024;12(2): 118. doi:10.3390/vaccines12020118. Pather S, Muik A, Rizzi R, Mensa F. Clinical development of variant-adapted BNT162b2 COVID-19 vaccines: the early omi­ cron era. Expert Rev Vaccines. 2023;22:650–61. doi:10.1080/ 14760584.2023.2232851. Pfizer/BioNTech COVID-19 Omicron-Modified Vaccine Options. [Internet]. Pfizer; BioNtech; 2022 [accessed 2023 May 23]. https:// www.fda.gov/media/159496/download. Zou J, Kurhade C, Patel S, Kitchin N, Tompkins K, Cutler M, Cooper D, Yang Q, Cai H, Muik A. et al. Neutralization of BA.4– BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with bivalent vaccine. N Engl J Med. 2023;388(9):854–7. doi:10.1056/NEJMc2214916. Statement on the antigen composition of COVID-19 vaccines. [Internet]. World Health Organization; 2023 [accessed 2023 May 24]. https://www.who.int/news/item/18-05-2023-statement-onthe-antigen-composition-of-covid-19-vaccines. ECDC-EMA statement on updating COVID-19 vaccines compo­ sition for new SARS-CoV-2 virus variants. [Internet]. European Centre For Disease Prevention And Control (ECDC) And European Medicines Agency (EMA); 2023 [accessed 2023 22 August]. https://www.ema.europa.eu/en/documents/other/ ecdc-ema-statement-updating-covid-19-vaccines-compositionnew-sars-cov-2-virus-variants_en.pdf. Updated COVID-19 vaccines for use in the United States Beginning in Fall 2023. [Internet]. United States Food And Drug Administration; 2023 [accessed 2023 22 Aug]. https://www.fda. gov/vaccines-blood-biologics/updated-covid-19-vaccines-useunited-states-beginning-fall-2023. European Medicines Agency. Comirnaty: eMA recommends approval of adapted COVID-19 vaccine targeting omicron XBB.1.5. 2023. Medicines and Healthcare products Regulatory Agency. MHRA approves Pfizer/BioNTech’s adapted COVID-19 vaccine (comir­ naty) that targets omicron XBB.1.5. 2023. Public summary of the risk management plan, comirnaty (COVID-19 mRNA vaccine). [Internet]. Pfizer; 2022 [accessed 2023 May 23]. https://www.swissmedic.ch/dam/swissmedic/de/ dokumente/marktueberwachung/rmp/covid-19_mrna_vaccine_ comirnaty-rmp-summary.pdf.download.pdf/Covid-19%20mRNA %20Vaccine_Comirnaty_riskmgtsystem-summaryrmpversion1. pdf. Prugger C, Spelsberg A, Keil U, Erviti J, Doshi P. Evaluating COVID-19 vaccine efficacy and safety in the post-authorisation phase. BMJ. 2021;375:e067570. doi:10.1136/bmj-2021-067570. FDA Approves First COVID-19 Vaccine. [Internet]. Food And Drug Administration; 2021 [accessed 2023 May 23]. https://www. fda.gov/news-events/press-announcements/fda-approves-firstcovid-19-vaccine. Meeting highlights from the Pharmacovigilance risk assessment committee (PRAC) 29 November - 2 December 2021. [Internet]. European Medicines Agency; 2021 [accessed 2023 23 May]. https://www.ema.europa.eu/en/news/meeting-highlightspharmacovigilance-risk-assessment-committee-prac-29november-2-december-2021. Ischemic Stroke. COVID-19 and Influenza in Adults Ages ≥ 65 Years: Interpretation & Next Steps. [Internet]. Twentyman E; 2023 [accessed 2023 Feb 28]. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-24/COVID-05Twentyman-508.pdf. European Medicines Agency. Report on pharmacovigilance tasks from EU member states and the European medicines agency (EMA) 2019–2022. 2023. EudraVigilance. [Internet]. European Medicines Agency; 2023 [accessed 2023 Feb 28]. https://www.ema.europa.eu/en/human- 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. regulatory/research-development/pharmacovigilance/eudravigi lance. United States Food and Drug Administration. Letter Of Authorization (Reissued). 2023. Guideline on Good Pharmacovigilance practices (GVP). Module VI – collection, management and submission of reports of sus­ pected adverse reactions to medicinal products (Rev 2). [Internet]. European Medicines Agency; 2017 [accessed 2023 May 23]. https://www.ema.europa.eu/en/documents/regulatory-procedural -guideline/guideline-good-pharmacovigilance-practices-gvpmodule-vi-collection-management-submission-reports_en.pdf. Chandler RE. Optimizing safety surveillance for COVID-19 vaccines. Nat Rev Immunol. 2020;20:451–2. doi:10.1038/s41577020-0372-8. Vaccine Safety Monitoring. [Internet]. United States Centers For Disease Control And Prevention; 2020 [accessed 2023 Feb 28]. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/ index.html. COVID-19 vaccine safety surveillance. [Internet]. United States food and drug administration; 2021 [accessed 2023 Feb 28]. https://www.fda.gov/vaccines-blood-biologics/safety-availabilitybiologics/covid-19-vaccine-safety-surveillance. VigiBase. WHO’s global database signalling harm and pointing to safer use. [Internet]. Uppsala Monitoring Centre; 2022 [accessed 2023 Feb 28]. https://who-umc.org/vigibase/vigibase-who-s-glo bal-database/. COVID-19 vaccine tracker. [Internet]. European Centre for Disease Prevention and Control; 2023 [accessed 2023 Feb 28]. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID19/vaccine-tracker.html#distribution-tab. COVID-19 vaccinations in the United States. [Internet]. US Centers for Disease Control and Prevention; 2023 [accessed 2023 February 28]. https://covid.cdc.gov/covid-data-tracker/#vaccina tions_vacc-people-booster-percent-pop5. Petousis-Harris H. Assessing the safety of COVID-19 vaccines: a primer. Drug Saf. 2020;43:1205–10. doi:10.1007/s40264-02001002-6. COVID-19 vaccines: Safety surveillance manual second edition. [Internet]. World Health Organization; 2021 [accessed 2023 May 1]. https://apps.who.int/iris/bitstream/handle/10665/345178/ 9789240032781-eng.pdf;sequence=1. COVID-19 AESI including status of associated Brighton case definitions, updated (October 2022). [Internet]. Brighton Collaboration; 2022 [accessed 2023 May 23]. https://brightoncolla boration.us/wp-content/uploads/2022/11/Updated-COVID-19AESI-list_Oct2022.pdf. Shemer A, Pras E, Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23:143–4. Cirillo N. Reported orofacial adverse effects of COVID-19 vac­ cines: the knowns and the unknowns. J Oral Pathol Med. 2021;50:424–7. doi:10.1111/jop.13165. Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021;147:739–43. doi:10.1001/jamaoto.2021.1259. Bertin B, Grenet G, Pizzoglio-Billaudaz V, Lepelley M, Atzenhoffer M, Vial T. Vaccines and Bell’s palsy: a narrative review. Therapie. 2022;78:279–92. doi:10.1016/j.therap.2022.07.009. Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, Gao L, Yu Q, Lam ICH, Chun RKC. et al. Bell’s palsy following vaccina­ tion with mRNA (BNT162b2) and inactivated (CoronaVac) SARSCoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22:64–72. doi:10.1016/S1473-3099(21)00451-5. Ahsanuddin S, Nasser W, Roy SC, Povolotskiy R, Paskhover B. Facial paralysis and vaccinations: a vaccine adverse event reporting system review. Fam Pract. 2022;39:80–4. doi:10.1093/fampra/ cmab068. Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D. HUMAN VACCINES & IMMUNOTHERAPEUTICS 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. et al. Transient facial nerve paralysis (Bell’s palsy) following intra­ nasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4:e6999. doi:10.1371/journal. pone.0006999. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T. et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory committee on immu­ nization practices — United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–82. doi:10.15585/mmwr. mm7027e2. Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Incidence, risk factors, natural history, and hypothe­ sised mechanisms of myocarditis and pericarditis following COVID-19 vaccination: living evidence syntheses and review. BMJ. 2022;378:e069445. doi:10.1136/bmj-2021-069445. Le Vu S, Bertrand M, Jabagi MJ, Botton J, Drouin J, Baricault B, Weill A, Dray-Spira R, Zureik M. Age and sex-specific risks of myocarditis and pericarditis following covid-19 messenger RNA vaccines. Nat Commun. 2022;13:3633. doi:10.1038/s41467-02231401-5. Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC. et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation. 2022;146:743–54. doi:10.1161/CIRCULATIONAHA. 122.059970. Husby A, Gulseth HL, Hovi P, Hansen JV, Pihlström N, Gunnes N, Härkänen T, Dahl J, Karlstad Ø, Heliö T. et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNA vaccination in four Nordic countries: population based cohort study. BMJ Med. 2023;2(1):e000373. doi:10.1136/bmjmed-2022-000373. Samimisedeh P, Jafari Afshar E, Shafiabadi Hassani N, Rastad H. Cardiac MRI findings in COVID-19 vaccine-related myocarditis: a pooled analysis of 468 patients. J Magn Reson Imaging. 2022;56:971–82. doi:10.1002/jmri.28268. Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, Hong R, Hanneman K. Myocardial injury pattern at MRI in COVID-19 vaccine–associated myocarditis. Radiology. 2022;304(3):553–62. doi:10.1148/radiol.212559. Clinical considerations: myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults. [Internet]. United States Centers for Disease Control and Prevention; 2022 [accessed 2023 Feb 23]. https:// www.cdc.gov/vaccines/covid-19/clinical-considerations/myocar ditis.html . Rafaniello C, Gaio M, Zinzi A, Sullo MG, Liguori V, Ferraro M, Petronzelli F, Felicetti P, Marchione P, Marra AR. et al. Disentangling a thorny issue: myocarditis and Pericarditis post COVID-19 and following mRNA COVID-19 vaccines. Pharm (Basel). 2022;15(5):525. doi:10.3390/ph15050525. Fairweather D, Beetler DJ, Di Florio DN, Musigk N, Heidecker B, Cooper LT Jr. COVID-19, myocarditis and pericarditis. Circ Res. 2023;132:1302–19. doi:10.1161/CIRCRESAHA.123.321878. Boehmer TK, Kompaniyets L, Lavery AM, Hsu J, Ko JY, Yusuf H, Romano SD, Gundlapalli AV, Oster ME, Harris AM. Association between COVID-19 and myocarditis using hospital-based admin­ istrative data — United States, March 2020–January 2020. MMWR Morb Mortal Wkly Rep. 2021 [March 2021–January 2021];70:1228–32. doi:10.15585/mmwr.mm7035e5. Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T. et al. Prevalence, characteristics, and outcomes of COVID-19–associated acute myocarditis. Circulation. 2022;145 (15):1123–39. doi:10.1161/CIRCULATIONAHA.121.056817. Barmada A, Klein J, Ramaswamy A, Brodsky NN, Jaycox JR, Sheikha H, Jones KM, Habet V, Campbell M, Sumida TS. et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibro­ tic myeloid response in SARS-CoV-2 mRNA vaccine–associated 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 15 myocarditis. Sci Immunol. 2023;8(83):eadh3455. doi:10.1126/ sciimmunol.adh3455. Moghimi SM. Allergic reactions and anaphylaxis to LNP-Based COVID-19 vaccines. Mol Ther. 2021;29:898–900. doi:10.1016/j. ymthe.2021.01.030. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–1. doi:10.1001/jama.2021.0600. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2. doi:10.1001/ jama.2021.1967. Maoz-Segal R, Shavit R, Kidon MI, Offengenden I, MachnesMaayan D, Lifshitz-Tunitsky Y, Niznik S, Agmon-Levin N. Late hypersensitivity reactions to the BNT162b2 SARS-CoV-2 vaccine are linked to delayed skin sensitization and prior exposure to hyaluronic acid. Life (Basel). 2022;12:2021. doi:10.3390/ life12122021. Wong KK, Heilig CM, Hause A, Myers TR, Olson CK, Gee J, Marquez P, Strid P, Shay DK. Menstrual irregularities and vaginal bleeding after COVID-19 vaccination reported to v-safe active surveillance, USA in December, 2020–January, 2022: an observa­ tional cohort study. Lancet Digit Health. 2022;4(9):667–75. doi:10. 1016/S2589-7500(22)00125-X. COVID-19 vaccines safety update 10 November 2022. [Internet]. European Medicines Agency; 2022 [accessed 2023 Feb 23]. https:// www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update /covid-19-vaccines-safety-update-10-november-2022_en.pdf. European Medicines Agency. Signal assessment report on heavy menstrual bleeding with tozinameran/comirnaty (COVID-19 mRNA vaccine). 2022. Edelman A, Boniface ER, Male V, Cameron ST, Benhar E, Han L, Matteson KA, Van Lamsweerde A, Pearson JT, Darney BG. Association between menstrual cycle length and COVID-19 vac­ cination: global, retrospective cohort study of prospectively col­ lected data. BMJ Med. 2022;1(1):e000297. doi:10.1136/bmjmed2022-000297. Kim MS, Jung SY, Ahn JG, Park SJ, Shoenfeld Y, Kronbichler A, Koyanagi A, Dragioti E, Tizaoui K, Hong SH. et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: A pharmacovigilance analysis using WHO international database. J Med Virol. 2022;94:1085–95. doi:10.1002/jmv.27424. Toepfner N, von Meissner WCG, Strumann C, Drinka D, Stuppe D, Jorczyk M, Moor J, Puschel J, Liss M, von Poblotzki E. et al. Comparative safety of the BNT162b2 messenger RNA COVID-19 vaccine vs other approved vaccines in children younger than 5 years. JAMA Netw Open. 2022;5:e2237140. doi:10.1001/jamanetworkopen.2022.37140. Mangat HS, Rippon B, Reddy NT, Syed AA, Maruthanal JM, Luedtke S, Puthumana JJ, Srivatsa A, Bosman A, Kostkova P. et al. Reported rates of all-cause serious adverse events following immunization with BNT-162b in 5–17-year-old children in the United States. PLoS One. 2023;18(2):e0281993. doi:10.1371/jour nal.pone.0281993. Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC. et al. Risks of myocarditis, pericarditis, and car­ diac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–22. doi:10.1038/ s41591-021-01630-0. COVID-19 vaccines safety update: 14 July 2021. [Internet]. European Medicines Agency; 2022 [accessed 2023 Feb 23]. https://www.ema.europa.eu/en/documents/covid-19-vaccinesafety-update/covid-19-vaccine-safety-update-comirnaty-14-july -2021_en.pdf. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293–302. doi:10.1016/S1473-3099(22)00320-6. 16 F. VAN DEN OUWELAND ET AL. 103. The Japan News. Japan OK’s pfizer vaccine for XBB.1.5 omicron variant. 2023. 104. Recommendation for the 2023-2024 formula of COVID-19 vac­ cines in the U.S. [Internet]. U.S. Food and Drug Administration; 2023 [accessed 2023 12 Aug 2023]. https://www.fda.gov/media/ 169591/download. 105. Pfizer and BioNTech dose first participants in the U.S. as part of global COVID-19 mRNA vaccine development program. [Internet]. Pfizer; 2020 [accessed 2023 Mar 3]. https://www.pfi zer.com/news/press-release/press-release-detail/pfizer_and_bion tech_dose_first_participants_in_the_u_s_as_part_of_global_ covid_19_mrna_vaccine_development_program.