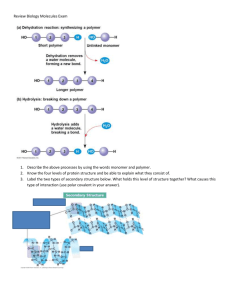
The general features of carbohydrates Carbohydrates are organic molecules composed of one or more monosaccharides They include sugar and polysaccharides Sugars include monosaccharides (single sugars) disaccharides (double sugars) Sugars taste sweet Sugars are small and soluble Sugars are the most important source of energy for many cells Two monosaccharides can be joined to make a disaccharide If monosaccharides continue to be added, a poly saccharide is formed Polysaccharides are large, complex molecules Usually they do not taste sweet Polysaccharides are insoluble Word equations for the formation of dissacharides glucose + glucose → Maltose + Water glucose + Galactose → lactose + Water glucose + Fructose → sucrose + Water These are all examples of condensation reactions Condensation reactions join two molecules together with the release of water Hydrolysis reactions split up two molecules using water (hydro=water; lysis=splitting). Form maltose by joining two alpha-glucose molecules. Highlight which parts of the molecules form water in this condensation reaction. Carbons 1 and 4 are linked via an oxygen atom to form a 1,4 glycosidic link (a covalent bond) Water is formed from the two hydroxyl groups (-OH) groups linked to carbon 1 and 4 Title Polysaccharides Monosaccharide monomers are linked together by condensation reactions to form disaccharides and polysaccharide polymers Task Draw a simple diagram of a linear chain of polysaccharides Draw a simple diagram of a branched chain of polysaccharides Polymers are formed in …………../polymerisation reactions. They can continue to create long chains of s………… (a carbohydrate). These building reactions are part of …………….. metabolism. Task 3 polysaccharides we will study are 1. Starch 2. Cellulose 3. Glycogen 2.3.U1 Monosaccharide monomers are linked together by condensation reactions to form disaccharides and polysaccharide polymers. Starch is only found in plants Starch is made from two polysaccharides: amylose and amylopectin Amylose is a chain of alpha-glucose monomers joined by 1-4 glycosidic bonds It is unbranched. The long chains of alpha glucose monomers forms a helix. Amylopectin is a chain of alpha-glucose monomers joined by 1,4 glycosidic bonds and some 1, 6 glycosidic bonds The 1, 6 glycosidic bond causes amylopectin to be branched. Explain how the structure of starch relates to function The function of starch is to store glucose. Starch is a very large molecule, so it is insoluble This means starch does not cause water to enter cells, where it is stored, (it has no osmotic effect). The helix is a compact shape to allow storage The branched structure of amylopectin, means that it has many ends. This allows starch to be more rapidly made or broken down. Title Glycogen – how structure suits function Structure • Glycogen has a similar structure to amylopectin • It is a chain of alpha-glucose monomers joined by 1,4 glycosidic bonds and 1,6 glycosidic bonds • There are more 1,6 glycosidic bonds than there are in amylopectin • Hence, glycogen is more highly branched Function • Glycogen is a large and compact store of glucose in mammalian muscle and liver cells • It is insoluble and so it will not cause water to move into cells where it is stored • More branches allow glycogen to be broken down very rapidly, to supply glucose for respiration • • • • Describe the structure of cellulose Cellulose is a chain of beta-glucose molecules joined by 1,4 glycosidic bonds It is unbranched Due to the position of the hydroxyl group (-OH) on carbon 1 in beta-glucose, every other glucose monomer must be rotated by 180 degrees to form 1,4 glycosidic bonds The long chain of beta glucose monomers is straight rather than in a helix Function • Groups of long, straight, cellulose molecules can line up close to each other • This allows many hydrogen bonds to form between polar –OH groups in neighbouring molecules • Cellulose molecules associate to form strong myofibrils • Microfibrils have great mechanical strength and form the cell wall of plant cells Compare the structure of starch, glycogen, and cellulose as viewed using Jmol software. Key features to view: Amylose – No branches and helix structure Amylopectin – Branches and 1,6 glycosidic bonds Glycogen – Similar to amylopectin but more branches Cellulose – No branches and straight molecule. Alternate flipping of glucose monomers in the polymer Title Name and describe the main types of lipids Type of lipid Description Triglyceride Composed of one glycerol and three fatty acids Joined by condensation reactions Examples include fats and oils Fats are solid and oils are liquid at room temperature (20oC) Are hydrophobic Phospholipid Like triglycerides but one fatty acid is replaced with a phosphate group Form the phospholipid bilayer of membranes Are amphipathic Steroids Characteristically contain four co-joined carbon rings in their structure (three hexagons and one pentagon) Include cholesterol and the male and female sex hormones Distinguish the structures of: 1. Saturated and unsaturated fatty acid 2. Monounsaturated and polyunsaturated fatty acids. Saturated fatty acid All the carbons in the hydrocarbon chain are linked by single bonds Unsaturated fatty acid At least one double bond in the hydrocarbon chain All carbons in the hydrocarbon More hydrogen could be chain are fully saturated with added (a process called hydrogen hydrogentatio Monounsaturated fatty acids Polyunsaturated fatty acids One double bond present in the Two or more double bonds hydrocarbon chain present in the hydrocarbon chain Title Lipids are better long-term stores of energy than carbohydrates Energy storage molecules in humans - Carbohydrate: glycogen is stored in liver and muscle cells - Lipids: triglycerides are stored in adipose tissue Feature Advantages of lipids over carbohydrates Energy released per gram Lipids release approximately twice the amount of energy per gram compared to carbohydrates Storage space An equivalent amount of energy will take up less storage space if lipids are used Energy stored per Although both are insoluble, unlike lipids, carbohydrates gram of body mass will attract more water molecules This increases the mass of stored carbohydrate In terms of body mass, this makes lipids able to store more than six times as much energy Lipids enable organism to transport large energy stores in a small mass This advantage of lipids is excellently demonstrated by migrating birds, which fly long distances without eating 2.3.S2 Determination of body mass index by calculation or use of a nomogram. 1. A man has a mass of 75 kg and a height of 1.45 metres. a. Calculate his body mass index. (1) BMI = mass in kilograms ÷ (height in metres)2 = 75 kg ÷ (1.45 m)2 = 75 kg ÷ 2.10 m2 = 35.7 kg m-2 a. Deduce the body mass status of 35.7 kg m-2 is above 30.0 (see table below) this man using the table. (1) therefore the person would be classified obese. BMI Status a. Outline the relationship between height and BMI for a fixed body mass. (1) Below 18.5 Underweight 18.5 – 24.9 Normal The taller a person the smaller the BMI; 25.0 – 29.9 Overweight 30.0 and Above Obese (negative correlation, but not a linear relationship) Title: Provide evidence supporting the statement that saturated fatty acids and trans fatty acids (trans – fats) increase health risks Saturated fatty acids Trans-fats Source Naturally found in animal fats and dairy produce Artificially made by adding hydrogen to polyunsaturated fatty acids Scientific evidence for health risk Positive correlation with coronary heart disease (CHD) in many population studies Positive correlation with coronary heart disease (CHD) in many population studies Conflicting evidence: - The Maasai people of East Africa have a high saturated fat intake but a low incidence of CHD One study showed that swapping 2% of the energy intake from trans fats with cis-unsaturated fats more than halves the risk fo CHD - All evidence points towards a casual relationship Other studies suggest there is a casual link between saturated fatty acids and CHD Evaluate evidence supporting the statement that saturated fatty acids and trans fats increase health risks Trans-fats and Saturated fatty acids Methods to obtain evidence - Population studies (epidemiology) Clinical studies Animal experiments Evaluation of evidence Correlation in population studies do not establish a casual link Sample size and genetic backgrounds should be considered Effects of other dietary components should be considered - There is evidence that some foods protect against CHD - There is evidence that foods with high glycemic index increase CHD Casual link between trans fats and CHD has been largely established. This has led to trans fats being banned in some countries. Casual link between saturated fatty acids and CHD is disputed