Heat and Temperature
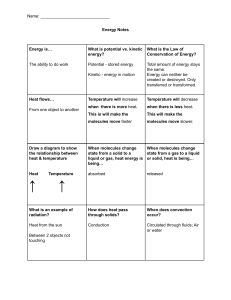
Heat is a form of energy. lt flows from one point to another due to temperature difference.
It is a scalar quantity, and its unit is Joule
(J).
Temperature is a physical quantity which determines the direction of flow of heat between
two objects. Heat flows from a higher temperature object to a lower temperature object"
@-+--6
TR
1;
/-:
"1,
''
The rate of heat flow is proportional to the temperature difference. Some physical
properties of objects depend on the temperature of the objects. These properties change if
the temperature is varied" Some examples of such properties are:
1,. Length of a liquid at constant cross-sectional area.
2. Volume of a gas at constant pressure.
3" Pressure of a gas at constant volume.
4" Resistance of conductors and semiconductors.
5.
Luminosity of an object.
Some of the properties mentioned above are exploited to construct thermometers.
The temperature of an object depends on the average kinetic energy of the molecules
it
is
composed of.
lnternal En.e[gy
\_,
Due to the intermolecular forces of attraction, all the molecules in an object have potential
energy. Moreover, these molecules also have kinetic energy, due to their random motion in
different directions. The summation of the potential energy and the average kinetic energy
is known as internal energy.
There is no intermolecular force between the molecules of an ideal gas. Thus, the internal
energy of an ideal gas is equal to the average kinetic energy of the molecules. lf heat or
thermalenergy is supplied from an externalsource, the internalenergy of the idealgas
changes.
tI
s
P
The graph above represents the action of heat energy
on an object's state
of matter.
Between initial time and t1 the supplied energy is used
to change the kinetic energy of the
molecules' Thus, the temperature of the solid obiect increases
from 0r to 02. Between t1 and
t2, the object changes its state from solid to liquid. Thus,
intermolecular separation
increases' Thisgiven energy is used to increasethe potentialenergyof
the molecules. since
the kinetic energy is constant, temperature remains unchanged. Between
tz and t3, the
temperature of the liquid increases, The given energy is used to
increase the kinetic energy
of the molecules. Between tg and ta, the provided thermalenergy
is used to increase the
potential energy of the molecules, as it changes state
from a liquid to a gas. As the kinetic
energy is constant, temperature remains unchanged. At ta,
the substance completely
changes to gaseous state. As the kinetic energy of the gas
molecule increases, temperature
increases with time.
The average kinetic energy of the molecules decreases if
the temperature is reduced.
-tr
\-)
Ts
G
P
LI
.P
o)
,z
)a
Te"nprr,"t re, (C)
At -273oC, the average kinetic energy of all substances become zero. The molecules in a
substance stop their vibration at this temperature. This temperature is known as absolute
zero temperature. By considering the lowest temperature to be zero, a new thermodynamic
scale was introduced, which is called
the Kelvin scale or the absolute temperature scale.
lf there is a temperature difference, heat flows from one object to another. When heat
flows, the average kinetic energy of the molecules decreases. Thus, the temperature of the
object gradually decreases with time. At the same time, the average kinetic energy increases
if the object receives heat. At any moment, when both objects in a system have the same
average molecular kinetic energy, heat flow stops.
Rate
of heat flow
x
change tntemperature.
The graph above represents the change in temperature of an object with time. The initial
temperature of the object is higher than room temperature. Due to the temperature
difference, heat flows out of the object and its temperature decreases. The gradient of this
graph represents the rate of temperature drop. lnitially, there is a large temperature
difference between the object and its surroundings, and thus, heat flows out of the object
to the surroundings. As the temperature of the object gets smaller, the temperature
difference with the surroundings decreases, and thus, the rate of temperature change
decreases. This is represented by the decreasing gradient.
To change the temperature of an object, thermal
energy is provided by an external source.
The amount of thermal energy required to change
the temperature of an object depends
on:
1. Mass of the object
2. Difference between initial and final temperature
For constant mass, the amount of thermal energy required
to change the temperature
directly proportionalto the difference between initialand final
temperatures.
H
is
x(0y_0)
HxA0
lf the temperature difference remains constant, the
amount of thermal energy required to
change the temperature is proportionar to the mass
of the object.
H
qm
Therefore,
H x. mA?
H = mcA?
The constant c is the specific heat capacity of the object.
lt is the property of the material.
Its unit is Jkg-1K-1.
specific heat capacity is defined as the amount of thermal
energy needed to change the
temperature of 1kg of an object by 10c (or 1K). lf heat or thermal
energy is provided at a
constant rate, it will take longer time to change the temperature
of a material with higher
specific heat capacity than of a material with lower specific
heat capacity.
The specific heat capacity of water is 4200 jkg-16t. Due
to this high specific heat capacity,
water takes a large amount of heat to change its temperature.
Oth Law
of Thermodvnamics
lf two objects of different temperature are placed close to each other, heat flows from one
object to the other, as long as there is a temperature difference between them.
i
d
sd-
,
P
When the two objects, A and B, reach the same temperature, heat flow stops. They are said
to be in thermal equilibrium. If the final common temperature of the objects A and
then the heat energy provided by object A is,
H4
= rttrc4 (0,
-
B is 0,
e)
The amount of energy received by the object B is,
Hs:mBcB@-0r)
The Oth law of thermodynamics represents the conservation of energy. According to this law,
the amount of energy provided by A is the same as the amount of energy received by
He=
Hs
macs(?2-0)=mBcB()-0)
moco92
-mnca9
-?(maca
-
maco? = mscB?
-
mBcs?,
-mrcs? = -TftscB?r-m4cs02
* mBcB) -
-(mBcs?1-t maca?2)
m4c402
e- mscs)1*
maCn * TftoCe
B.
Experiment to Determine Specific Heat Capacitv of Solids and
Liquids
r-t'
t
r--l,t
lt h*r*wb"
11^ut*r,d<n
Iv.ttouir-r
tt<.al01
CoVPA
Gvla^^pn
6oh1
Vato**
Fi?'r^c ta'
Figure 1a shows the apparatus which is used to identify the specific heat capacity of liquids.
lnitially, the mass of the liquid is measured using an electric balance, and the temperature of
the liquid is measured using a.thermometer before the circuit turned on. When the switch is
closed, a stopwatch is started, and after some time before turning off the circuit, ammeter
and voltmeter readings are taken, and the final temperature is recorded when the
temperature reaches a steady value.
H
=VIt
H=mc(?r-0i)
Therefore,
mc(er-ot)=vtt
VIt
m(07
- 0)
Where, c is the specific heat capacity'
The same technique is used to measure the specific heat capacity of solids. The only
difference is that oil is present between the thermometer and the solid object, which
prevents damage to the thermometer in case of uneven heating.
Kinetic Theorv of Gas
An idealgas is modeled according to the following assumptions:
L. The gas is made up of identical particles called molecules.
2. These molecules are vibration in random directions. During their vibration, they
3.
4.
5.
6.
collide with each other and also with the walls of the container of the gas.
Their collisions are perfectly elastic. The time of collision is very small.
There is no intermolecularforce of attraction between the idealgas molecules.
The total volume of a gas molecule is negligible compared to the entire volume of
the gas.
They follow root-mean-square speed
Root-Mean-Square Speed (RMS Speed)
/
l vl\
| .rjl
The container contains n number of molecules, which are moving randomly in different
directions, with a wide range of speeds. Since the container has a large number of gas
molecules, their average velocity is zero.
Average speed,
cavg
=
€1*
c2
+ ca + ...1
cn
Mean-square speed,
(c2l =
cr2+cr2+crz+,..+cnz
Root-mea n-square,
,Rr')
' + ,r' * ,..r cr2
=
RMS speed has a non-zero
magnitude. lt is considered to be the average speed
of the gas
molecules in a sample. so, the total kinetic energy
of gas the gas molecules in a sample is,
_
Ex =
1
^+ 1 "1.
^ ...+
7mcrz Z*rr, + )mcz2 +
i,^rr,
1
1
Ex =
2m(c12
* ...+ cnz)
+ crz + czz
We know that,
+
c12
\c')
c2
'
+
rr' *...1
cn2
n
n(c2) = ctz
* ...* cn2
*
crz + cr2
+
rr, + cr2 +...+
Therefore,
E* =
1
1m(cr,
cn2)
u* =)..mn(cz)
The average kinetic energy depends on the mean
square speed, which is proportionalto the
absolute temperature of the gas.
E*orn
Exoun
x
T
= kT
Bovle's Law
Boyle's law is described as the relationship between pressure and volume of a gas at
a
constant temperature.
Boyle's law states that the volume of a fixed mass of gas is inversely proportional to the
pressure.
, oF1
v--Pk
V1P1:
V2P2
Experiment to Determine Bovle's Law
?rcssu11- Qawge
Air is trapped in a vertical cylindrical tube filled with oil. The vertical tube is connected to a
pressure tube and a pressure gauge. By using a pump, the pressure is gradually iricreased. At
large values of pressure, the volume of air particles decreases. Pressure is measured using
the pressure gauge, and the volume is calculated by measuring length of air in the vertical
tube using the metre rule, and multiplying it by the circular cross sectional area of the
cylindrical tube. For each value of pressure at constant intervals, the corresponding value of
volume is noted. A volume against pressure graph is plotted according to the data.
PRECAUTION: in this experiment, the pressure must be changed slowly. This is done to
prevent change in temperature, as temperature is the controlled variable. The mass of gas,
or the number of particles of gas under observation should be kept constant.
Several volume against pressure graphs are plotted at different temperatures each. All the
graphs will follow the same pattern, but the graph of higher temperatures will be
completely above those of the lower temperature.
Charles's Law
This law represents the relationship between temperature and volume at constant pressure.
This law states that the volume of an ideal gas is directly proportional to the absolute
temperature, provided that the pressure and the mass of gas remain constant.
VxT
V=kT
V
,.
--K
T
V\
-=T!
V2
Tz
Experiment to Determine Charles,s Law
&.qillav1
T*b
A thin cylindrical capillary tube sealed at one end is plugged at the centre
with a drop of
concentrated sulphuric acid. A ruler is attached to the capillary tube, so that the height h
from the sealed end to the drop of acid can be measured. The setup is immersed in a beaker
containing oil, and a heater is used to heat the beaker. A thermometer is used to measure
the temperature. The volume of gas in the capillary tube blocked by the plug of acid is
measure using the formula,
V=
rrzh
Where, r is the cross-sectional radius of the cylindrical capillary tube, and h is the height for
the sealed end to the plug, measured using the ruler.
For temperature at suitable interval, the corresponding value of volume is measured.
Temperature
Volume
T1
V1
T2
Yz
Tn
d
:5
TenTerulvw
GrE"t)
lf a graph is plotted taking the temperature in degrees Celsius, all the
straight lines for
different gases have constant gradient (different for each gas), but none
of them pass
through the origin' For all gases, their lines intersect the temperature axis (horizontal
axis)
at -273oc. This temperature is called absolute zero. lf the temperature is taken
in Kelvin (the
absolute temperature scale), this line will have same gradient, but will pass
through the
origin.
ol
t
=
To.r+*adww
(fot";*)
Pressure Law
This law states that the pressure of a gas at constant volume is
directly proportional to its
absolute temperature, provided that mass of gas remains constant.
P
xT
P=kT
P,
- P,
T7 Pz
Experiment to Verifv Pressure Law
\to*vrq*,ar
?wsuta fia*Xo
FlaSk go"|si^inX
*;r
0il
In this experiment, the volume of gas is kept constant. lts pressure can be measured using a
pressure gauge. A heat source is used to increase to temperature of the gas. This
temperature is recorded using a suitable thermometer. By using the heat source, the
temperature is gradually increased.
Temperature
Pressure
Tr
P1
Tt
P2
Tn
Tn
Tevwrr.",u*uAs. (1ry;tvf$
lf the temperature is taken in degrees Celsius, all the straight lines of different gases have
constant gradients (different for each gas), but none of them pass through the origin, All the
lines intersect the temperature axis (horizontal axis) at -273oC. At this temperature, the
pressure of gases becomes zero (theoretically).
As the pressure of the gas becomes zero, the
gas molecules stop vibrating at this temperature.
By combining this
equation for constant mass, we can write,
P\Vl _
P2V2
TL
T2
PV
-n
T-n
ln this equation, R is a constant, called the molar gas constant.
R = 8.31,
Jmol
1l(-1.
PV=RT
This equation is valid for one mole of gas.
For n moles of gas, the equation becomes,
PV = nRT
Where, n is the number of moles of gas.
ldeal Gas Equation
P1V7
Tt
_
P2V2
T2
PV
_ - t,
-^
T
PV=kT
(For one mole of gas)
ln the equation above, k is a constant called the molar gas
constant.
For n moles of gas,
PV =nkT
The constant k is replaced with the alphabet R, where
PV =nRT
R
= g.3L Jmol-1K-1
For N number of molecules,
N
TL=-
N"
Where, n is the number of moles,
constant. (N. = 6.023x1023)
is
the number of molecules, and N.
N
PV
RT
No
R
PV
is Avogadro's
NT
No
Here,
R
*r=
k
Therefore,
PV
=
kNT
Where, k is the Boltzmann constant, and k = 1.38x10
23.
The figure above represents a gas container containing n number of molecules. The length
of each side of the container is /. Hence, the cross-sectional area of the container is /2 and
the volume of the container is /3. c represents the velocity of the molecules inside the
container. The velocity of each particle can be resolved in three dimensions.
crz=c7r2+cru2+cr, 2
cL''c.oandcrzarethecomponentsofvelocityinthedirectionx,
travel between two vertical wall is
t, where,
yanr)z.thetimetakento
d
The number of collisions within
this time is one. Thus, the change
in momentum within this
time is,
AP.
/ -t
c_
ITLCI*_Tnc1-
F:^^-t
t
,-
-zmcr,
t
,-
-2mcr,
2l
C.Lx
-m("*)'
' L
The method used above is shown
below' The collision is considered to
be completelyelastic,
and so no kinetic energy is lost.
Be{""e
Cotlisi"n
+?<
e-----(,/-\
A++t^
Co\tisian
- m(u-u)
t
- m(-x - x)
J,I-
t
Zmx
r- = -t
Pressure on the Wall
F
D_
I
A
--
\2
-m(c,
p-_____:_:1:_i12
I
D--'x' -m(c.
l3
n
P
-
)2
-m(c,,)'
V
m
- _vG4)'
Calculation of Total Pressure for N number of Molecules
,=ry)_
r:2r:2r;2.12
P=
-^lT) + +W{
m
...+ cn*2'1
'y
i' (rr,' czt a czt +
P
=T N '\cr'l
From root-mean-square speed equation,
\cr) = \crrl + er2l + (cr2l
(c,,1 = \cr)
-
\crr)
-
(c,21
Assuming that c*, cy, ohd c, are equal,
\c') = 3\cr'l
(c*rl=1*(.r)
Therefore, putting this value in the equation,
p
m
=;v5x rV x ix (rrl
D-
mN
k2)
3V
lf density is given,
p =:.pN\c2)
We know that,
PV = kNT
,=
Therefore, substituting this value for
P
KNT
U
in the previous equation,
kNT _mN(cz)
V3V
m(czl = 3kT
l' ^k'J = 1' kr
c _
,x
-3i . nt,rr
Therefore,
E*
xT
Maxwell
- Boltzmann Distribution
o)
s
tY
o
x
+s
s
-cI
23
lf a sample of n number of gaseous molecules vibrates or moves within a wide range of
speed, the Maxwell - Boltzmann distribution graph represents the number of gas molecules
with different speeds. The speed corresponding to the peak of the graph represents the
most probable speed of the niolecules. The area under the graph represents the total
number of gas molecules.
ln the graph above, the shaded area represents the number of molecules with speeds
ranging from v1 to v2. lf the temperature is increased, the average kinetic energy of the gas
molecules increases. Thus, the peak of the graph shifts toward a higher speed or higher
energy region. But, the peak becomes lower, which indicates a smaller number of molecules
at the most probable speed or energy.
bqy
<3
)a,
T,)T,
St.l
!q
2=Z
The areas under the two graphs are the same, which indicates equal number of molecules.
ln liquids, the molecules also have a wide range of kinetic energies. lf their kinetic energy
exceeds the minimum value, it is capable to leave the liquid surface. This process of leaving
the liquid surface is called vaporization. According to this graph, at higher temperatures, the
number of molecules with sufficient energy to evaporate is large. Thus, the rate of
evaporation increases with temperature.