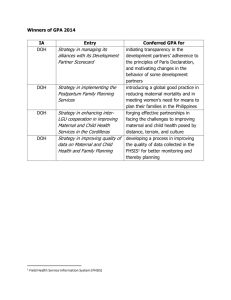
LABORATORY MANAGEMENT Lesson 3: Regulation, Accreditation, And Legislation in the Philippines RA 4688 - an act regulating the operation and maintenance of clinical laboratories and requiring the registration of the same with the department of health, providing penalty for the violation thereof, and for other purposes - signed into law by President Ferdinand Marcos Sr. C. Clinical Laboratory (CL) - facility involved in pre-analytical, analytical, and post-analytical procedures, where tests are done on specimens from the human body to obtain information about the health status of a patient AO 2021-0037 - one of the main thrusts of current health sector reforms under FOURmula One (F1) for health is regulation - The main objective of regulatory reforms is to ensure access to quality and affordable health products, devices, facilities and services, especially those commonly used by the poor - Physicians utilize lab workups in aid of diagnosis and management of patients - Accuracy of lab results are important in assuring and improving the quality of patient care - RA 4688 s. 1966 mandated the DOH to look after public welfare by effectively enforcing and updating the current regulations to improve laboratory - Scope and Coverage - the AO shall apply to all individuals, agencies, partnerships or corporations that operate clinical laboratories in the PH performing examination and analysis of samples of tissues, fluids, secretions, excretions, or other materials from the human body that would yield relevant lab information, which physicians use for the prevention, diagnosis, and treatment of diseases, and the management and promotion of personal and public health - Government clinical laboratories, doing microscopy work only for specific DOH programs such as but not limited to malaria screening, acid fast bacilli microscopy, tests for sexually transmitted diseases D. Department of Health – License to Operate (DOHLTO) - formal authorization issued by the DOH to an individual, partnership, corporation, association or any government agency/unit seeking to perform laboratory tests in compliance with the requirements in this order DEFINITION OF TERMS I. National external quality assessment scheme (NEQAS) - an EQAP activity conducted by the national reference laboratories to assess the quality of performance and accuracy of the results of laboratories A. Applicant - refers to any natural juridical person, government instrumentalities/agencies, partnership, corporation or agency seeking a license to operate and maintain a clinical laboratory B. Assessment Tool - checklist which prescribes the minimum standards and requirements for licensure of a clinical laboratory E. Department of Health – Permit to Construct (DOHPTC) - permit issued by DOH through HFSRB or Center for Health Development-Regulation, Licensing, and Enforcement Division (CHD-RLED) to an applicant who will establish and operate a hospital or other health facility F. External Quality Assessment Program (EQAP) - program where participating CL are given unknown sample for analysis G. Initial Application - applications by newly constructed health facilities or those with changes in the circumstances of the facilities such as but not limited to change of ownership, transfer of site, increase in beds or for additional services beyond their service capability and major alterations or renovations H. Mobile Clinical Laboratory (MCL) - lab testing unit capable of performing limited CL diagnostic procedures - moves from 1 testing site to another and has a DOHlicensed CL as its main laboratory J. National reference laboratory (NRL) - highest level of lab in the country performing highly complex procedures - includes confirmatory testing (not commonly performed by the lower level of labs) K. Physician’s Office laboratory (POL) - doctor’s office/clinic wherein CL examinations are performed for the purpose of monitoring the doctor’s patients only - no official results shall be issued - POL w/in the premises of a DOH- regulated facility shall be under the supervision of the CL L. Point of Care testing (POCT) - diagnostic testing done at or near the site of patient care rather than in the CL FUNCTION 1. Clinical Pathology M. Satellite clinical laboratory (SCL) - extension of the main CL located w/in the facility’s compound or premises 2. Anatomic Pathology N. Referral tests - CL tests that are either sent-out or outsourced to other DOH licensed CL with the same or higher service capability Permit to Construct 1. filled out application form from DOH-PTC (manual or online) shall be submitted to the DOH regulatory offices, as specified in Section V. B of this order 2. DOH-PTC shall be required for construction of new CL and for renovation or expansion of existing CL, including change in ownership and transfer of location 3. application shall be processed in accordance w/ procedural guidelines set SPECIFIC GUIDELINES CLASSIFICATION BY OWNERSHIP Government - operated by the national government, LGU, or any other government agencies Private - privately-owned - funded by donations or corporations, and individuals by organizations, INSTITUTIONAL CHARACTER Institution based - lab located w/in the premises and operates as part of a DOH licensed health facility Non-institution based - lab that operates independently and is not attached to any DOH licensed health facility CLINICAL LAB SECTIONS AND EXAMS FOR CLINICAL AND ANATOMIC PATHOLOGY (DOH) PROCEDURAL GUIDELINES License to Operate (LTO) 1. Submit an accomplished application for to HFSRB/CHD-RLED in accordance w/ current DOH guidelines (manual or through online licensing and regulatory system (OLRS)) once it is fully functional 2. Complete application for DOH-LTO shall consist of the following: - notarized acknowledgement - proof of ownership (Ex. DTI registration) - accomplished self-assessment tools - health geographic form 3. the HFSRB/CHD-RLED representative reviews the application and conducts on-site assessment of the lab 4. if the lab is not fully compliant w/ the licensing requirements, the HFSRB/VHD-RLED shall provide a written report outlining the lab’s deficiencies 5. The DOH-LTO, whether initial or renewal, shall only be issued after the HFSRB/CHD-RLED, in accordance with the current DOH guidelines, has determined that the laboratory is fully compliant 6. Submitted complete applications that are not processed within twenty (20) days by the HFSRB/CHDRLED, in accordance with the current DOH guidelines, due to force majeure, shall automatically be granted the LTO, and a post-licensing visit 7. Only DOH licensed CL identified by the program and has already secured a certificate of “Certified rHIVda Confirmatory Laboratory (CrCL)” from the NRLSLH/SACCL or its designated regional counterpart, shall be allowed to apply for a license CrCL 8. A DOH-licensed hospital-based tertiary CL, already certified by the NSRC, may apply as a G6PD Deficiency confirmatory laboratory to HFSRB 9. For institution-based CL, the One-Stop Shop (OSS) Licensing System, pursuant to Administrative Order No. 2018-0016 dated June 4, 2018, titled “Revised Guidelines in the Implementation of the One-Stop Shop Licensing System,” shall be 10. The DOH-LTO is non-transferable and a new application for DOH-LTO shall be required in case of change of ownership or transfer of location 11. The HFSRB/CHD-RLED, in accordance with the current DOH guidelines, shall be notified in writing of any change in management name, ownership, or headship or laboratory personnel. Failure to notify of any substantial change in the condition of the laboratory, i.e. changes in the physical plant, equipment, or personnel, in writing within fifteen (15) days, may be a basis for the suspension or revocation of license 12. Different branch(es) of a CL, even if owned by the same entity shall secure separate DOH-LTO 13. Application for DOH-LTO shall be in compliance with AO No. 2019-0004 dated April 30, 2019, titled “Guidelines on the Annual Cut-off Dates for Receipt of Complete Applications for Regulatory Authorizations Issued by the Department of Health.” 14. The DOH-LTO shall be placed in an area that can be readily seen by the public, at all times. Certificate of Registration (COR) - required for research and teaching labs - applicants shall submit an accomplished registration form together w/ the necessary attachments to HFSRB, which includes Annex for services - the applicant shall be required to pay a non-refundable fee before submission of the requirements as part of complete application Validity - the DOH-LTO is valid for one (1) year - COR for CL that is operated and maintained exclusively for research and teaching purposes shall be required to register with the DOH-HFSRB every three (3) years Fees - all fees shall follow the prescribed fees by DOH - all fees/checks shall be paid to the order of DOH Central Office/CHD cashier, whichever is applicable in person, through postal money order or online payments approved by the DOH Monitoring 1. Authorized representatives from the HFSRB/CHDRLED in accordance with the current DOH guidelines, may conduct unannounced on-site visits of licensed CL and registered research and teaching to monitor and document the continuous compliance of the CL to the set standards 2. If upon monitoring visit, the CL is found to be violating any of the rules and regulations stated herein relative to its operation, the HFSRB/CHD-RLED in accordance with the current DOH guidelines, may immediately impose preventive suspension 3. CL that are operated and maintained exclusively for research and teaching purposes shall not issue official results for diagnostic purposes. They may be monitored to ensure that they are not operating beyond allowed capabilities. ROLES AND RESPONSIBILITIES Health Facilities and Services and Regulatory Bureau - set standards for the regulation of CL and strictly enforce the provisions of this order - disseminate regulatory policies, standards and forms for information and guidelines of the DOH-CHDs - provide consultation and technical assistance to stakeholders, including regulatory officers from the DOHCHDs in line with the regulation of CL Center for Health Development - strictly enforce the provisions of this order - submit quarterly report on suspension/revocation/ cease and desist order issued on CL not later than 15th day of the following month after the covered quarter - provide consultation and technical assistance to stakeholders in line w/ he regulation of CL - respond promptly to complains relative to the operation of CL under its National Reference Laboratories - provide laboratory reference/referral services for confirmatory testing - Train laboratory personnel and recognize other training institutions - Maintain the National External Quality Assessment Scheme (NEQAS) - Perform technical evaluation of reagents and diagnostic kits DOH-Licensed Clinical Laboratories - continuously comply w/ rules and regulations - participate in EQAP administered by the NRL or any other agencies - in times of pandemic or public health event, be mandated to submit timely reports and data VIOLATIONS, SANCTIONS, AND APPEAL - a CL shall be sanctioned and penalized by the HFSRB/CHD Director upon violation of any of these guidelines and its related issuances and laws, or upon committal (commission/omission) of prohibited acts (Annex C) by the persons owning or operating the CL, and/or the persons under their authority - For non-institution-based CL that are not under the OSSOLS, the following are the penalties and sanctions that shall be imposed for the commission of any of the violations in this Order and other relevant issuances: I. 1st offense: stern warning II. 2nd offense: Php 30,000 III. 3rd offense: Php 50,000 IV. 4th offense: Revocation of DOH-LTO - Any person who operates a CL without securing the necessary DOH-PTC and corresponding DOH-LTO shall be issued a Cease-and-Desist Order (CDO) and shall pay the administrative penalty of Fifty thousand pesos (Php50,000.00) - Section 4 of Republic Act No. 4688 shall still be imposable aside from the administrative penalty provided in this Order - In case of complaints, the CL, upon receipt of such by HFSRB/CHD- RLED shall be given due process wherein an investigation shall be conducted and the appropriate sanctions for its violation/s. A 60-day preventive suspension may be given to the CL during the investigation depending on the seriousness of the violation. - Any CL or any of its personnel not amenable with the decision of the HFSRB/CHDRLED may, within ten (10) days after the receipt of notice of decision, file a notice of appeal to the Head of the Health Regulation Team (HRT). All pertinent documents and records of the appellant shall then be elevated by HFSRB/CHD-RLED to the HRT. The decision of the Head of the HRT, if still contested may be brought on a final appeal to the Secretary of Health, whose decision shall be final and executory. - Any person authorized or licensed to conduct clinical laboratory tests, who issues false or fraudulent laboratory test results knowingly, willfully or through gross negligence shall not be allowed to own, manage, operate, or be an analyst of a CL. TRANSITORY PROVISIONS A. All existing licensed CL shall be given three (3) years to comply with the physical plant requirements from the date of effectivity of this Order. B. All existing licensed CL shall be given two (2) years to fully offer the additional services for each category with corresponding personnel and equipment from the date of effectivity of this Order. C. For new CL, this Order shall be immediately applicable. D. For CL currently headed by Anatomic Pathologists with an associate Clinical Pathologist or Clinical Pathologists heading tertiary CL with Anatomic Pathology services, such headships shall be retained until his/her eventual retirement, resignation or replacement. Thereafter, all CL shall be headed by a pathologist certified in Clinical Pathology by the Board of Pathology of the Philippine Society of Pathologists, except for tertiary CL with anatomic pathology service which shall be headed by a pathologist certified in both Anatomic and Clinical Pathology ANNEX A Physical Plant - Every clinical laboratory (CL) shall have an adequate space for its operation to safely, effectively and efficiently provide services to clients. A. The CL shall conform to all applicable local and national regulations for the construction, renovation, maintenance and repair of CL. B. The laboratory shall conform to the required space for the conduct of its activities. Personnel, fixtures, equipment, sink, etc. shall also be considered. Minimum area requirements for each are listed in Annex D. C. There shall be well-ventilated, lighted, clean, safe and functional areas based on the services provided. D. There shall be a program of proper maintenance and monitoring of physical plant and facilities. E. There shall be policy guidelines on laboratory biosafety and biosecurity which includes risk assessment that will serve as the basis of biosafety level required for the specific CL. F. There shall be an area for confirmatory testing for Rapid HIV Diagnostic Algorithm (CrCL) and Glucose6-Phosphate Dehydrogenase (G6PD) Deficiency which may be a section, unit, or division integrated in a DOH licensed CL, if applicable. Personnel - Every CL shall have an adequate number of trained personnel, depending on the workload, to provide safe, effective and efficient services to clients. - Head of the Laboratory (HOL) - Registered Medical Technologist (RMT) - Support Staff - POCT Coordinator (if applicable) - POCT operator (if applicable) MCL Personnel - MCL shall has its own set of personnel, which includes the following but not limited to: a. Registered Medical Technologist — number will depend on the anticipated workload. b. Support staff such as, but not limited to, driver and laboratory technician. EQUIPMENTS/INSTRUMENTS/REAGENTS/ GLASSWARES/SUPPLIES A. There shall be available and operational equipment/machines/devices to provide the laboratory examination that the laboratory is licensed for. B. There shall be a calibration, preventive maintenance and repair program for every equipment/machines/ instruments/device in the DOH licensed CL. C. There shall be a contingency plan in case of equipment/machines/devices breakdown and malfunction. D. There shall be adequate available reagents, glassware and supplies for the laboratory examinations. E. There shall be an inventory control of the reagents, glassware and supplies. F. The reagents, glassware and supplies shall be properly stored under the required conditions. G. The machines/devices, reagents and test kits that are used in the CL and MCL as well as POCT shall be approved by the Philippine Food and Drug Administration and validated by the proper government institutions (e.g. National Reference Laboratory). H. The MCL shall have its own set of functional, and operational equipment, as well as its own set of supplies. Service Delivery - The services provided by the CL shall ensure quality and safety to clients, to its personnel and to the general public Information management - Every CL shall maintain a system of communication, recording, reporting and releasing of results Quality Improvement A. Every CL shall establish and maintain a system for continuous quality improvement activities. There shall be an Internal Quality Assurance Program which shall include: 1. An Internal Quality Control Program for technical procedures. 2. An Internal Quality Assurance Program for inputs, processes and outputs. 3. A Continuous Quality Improvement Program covering all aspects of laboratory performance. B. The CL shall participate in External Quality Assessment Program (EQAP) that may be administered by a designated NRL or other local and international EQAP approved by the DOH. C. A periodic assessment shall be conducted by representatives from the top management, clinical laboratory, clinical departments and nursing service, to evaluate the policy of the CL on POCT. Referral of Laboratory Examinations - Every CL shall ensure the quality of services provided through an agreement, or its equivalent, to a DOH licensed CL performing the laboratory services needed. Environmental Management - Every CL shall ensure that the environment is safe for its patients and staff, including the general public. Clinical Sections and Required Equipment LABORATORY MANAGEMENT Lesson 4: Safety SAFETY Introduction - All healthcare facilities must have facilities to address the various types of exposure - Infectious biologicals - Toxic chemicals - Radioactive materials - Ergonomic/environmental hazards - Fire safety - Epidemics STRATEGIES TO CONTAIN HAZARDS BIOLOGICAL HAZARDS Sources - Bacteria - Virus - Parasites - Prions (small viral particle that can cause infection) Mode of exposure/transmission - Ingestion - Inoculation (needle prick) - Tactile contamination - Inhalation of infectious material from patients (sputum, saliva) - Aerosol dispersion Public Exposure - Aerosolized infectious material - Improperly processed blood products - Inappropriately disposed waste products (cause community-acquired infection) Contagious outbreaks CDC AND OSHA’S ROLE - Universal Precautions (1980s) - Occupational exposure - “reasonably anticipated skin, eye, mucus membrane, or percutaneous contact with blood or other potentially infectious materials that may result from the performance of an employee’s duties PRACTICES THAT SHOULD BE AVOIDED - Mouth pipetting - Food or drinks consumption - Smoking - Cosmetics application - Potential needlestick situations - Unprotected skin, membranes, or open cuts CHEMICAL HAZARDS - OSHA - Hazard Communication Standard, Chemical Hygiene Plan SOURCES OF AEROSOL CONTAMINATION - Flaming or inoculating loop - Spills on lab counters - Expelling a spray from needles - Centrifugation of infected fluids PROPER LABORATORY ATTIRE - Gloves - Masks - Protective eyewear - Face shield - Laboratory coat - Observe proper handwashing FOR DECONTAMINATION OF SOILED AREAS - Safety Data Sheet Hazard Communication plan for employees ERGONOMIC HAZARDS Cumulative Trauma Disorders Other names - Repetitive strain injuries - Repetitive motion disorders - Overuse syndrome - Work-related musculoskeletal disorders Proper chemical storage - specific chemicals should be stored in safety cabinets or under hoods - separate storage of acids and bases Risk Factors - Repetitive motions - Forceful exertions - Awkward postures - Back Flexion - Back Exhaustion - Twisting about waist - Lateral bending - Static postures - Mechanical compression of soft tissues - Fast movement of body parts - Vibration - Mental stress - Lack of sufficient recovery time Causes of Mental Stress - Work-related stress - Relationship stress - Financial stress - Parenting stress - Health stress - Life changes Cumulative Trauma Disorders – Laboratory Personnel - Can cause Carpal Tunnel syndrome, tendonitis, tenosynovitis - Repetitive pipetting - Working at a microscope - Resting the wrists/arms on sharp edges Cumulative Trauma Disorders – Management - Proper posture when sitting or standing - Minimize eye strain situations - Hand, arm, back, leg, neck exercises - Take frequent 1-2 minutes break LABORATORY MANAGEMENT Lesson 5: Optimizing Laboratory Workflow and Performance 3 PHASES OF TESTING PROCESS - provides info about how many containers are received w/in a specified interval 1. Preanalysis - all the activities that take place before testing, such as test ordering and sample collection - Steps: clinical need, order, collect, transport, receive, sort, prepare/centrifuge, uncap (if needed), aliquot 2. Analysis - consists of the laboratory activities that actually produce a result, such as running a sample on an automated analyzer - Steps: load sample on analyzer, add sample/reagents, mix, incubate, detect, reduce data, produce result, review result, repeat test (if necessary), release result 3. Postanalysis - comprises patient reporting and result interpretation - Steps: recap tube, postprocessing storage, report result, access result, interpret result, integrate w/ other clinical info, clinical action Steps in the testing process categories - Testing phase - Role (responsibility) - Laboratory technology DATA COLLECTION TECHNIQUES A. Sample and Test Mapping - analyze the distribution of samples and tests over time - the goal is to identify overall workload patterns to assess whether resources are appropriately matched to needs and whether turnaround time or other performance indicators can be improved - by mapping samples and tests and relating them to TAT and staffing, a lab can identify production bottlenecks and alter workflow to achieve better outcomes B. Tube Analysis - “tube labor” includes sorting and centrifuging; aliquoting; racking, unracking, loading, and unloading samples on analyzers; retrieving tubes for add-on tests; performing manual dilutions or reruns (depending on instrument); and storing tubes - helps to analyze how many additional tube-related tasks have to be done - includes the number of containers other than tubes (e.g., fingerstick collections that may require special processing or aliquoting) and the number of reruns (i.e., repeats) needed as the result of instrument flags and/or laboratory policies C. Workstation Analysis - Goal: understand where, when, and how the work is performed - review of lab test offerings should be done during workstation analysis - Workstation: is one physical location (e.g., a fully automated analyzer or group of analyzers, such as hematology cell counters, a chemistry workcell, or an automated track that includes preanalytic processing, analytic modules, and postanalytic storage) - all manual or semiautomated chemistry tests may be grouped into a workstation, even though testing might actually be performed at different sites or using different equipment around the laboratory D. Instrument Audit - better understand how each analyzer is used, its associated costs, and what potential opportunities might exist to improve performance E. Test Menu - this option can be easily overlooked if one focuses only on how to improve the way existing tests are performed instead of analyzing how to best meet clinician needs - Just because a laboratory can perform a test does not mean that it should - Ex: if a test (e.g., viral load) is performed only once or twice a week and requires dedicated equipment and space, training, and/or labor, it may make more sense to send it to a reference laboratory where it is performed more frequently and, often, at a lower cost. Sometimes, the best way to improve turnaround time and/or lower the cost of a test is not to perform it - Task mapping should be an ongoing activity and should also be undertaken whenever one contemplates adding a workstation, test, new technology, or any significant change to a laboratory process - Mapping also helps compare processes before and after change F. Processing Mode and Load Balancing - can affect both the cost and the timeliness of testing - When grouped into batches, samples are run at specific intervals (e.g., once a shift, once a day, every other day) or whenever the batch grows to a certain size (e.g., every 20 samples) - Batch processing: is often less expensive than continuous processing because the setup costs (quality control, labor, etc.) are spread over many specimens - produce less timely results; limitation of the instrument that is used - Continuous processing is facilitated by load balancing, a technique that distributes work evenly among analyzers and spreads testing over a longer period to better match instrumentation throughput E. Interviews - This exercise provides an opportunity for staff to participate in analyzing workflow and improving performance - It also identifies issues that would not be readily apparent from data collection alone - Interviews are particularly valuable in understanding what occurs outside the laboratory - Test ordering patterns or habits can have a significant impact on a laboratory’s ability to meet clinician needs. - Visits to patient care units and discussions with nursing unit staff can identify preprocessing improvements that cost little to implement but save considerable money downstream. G. Task Mapping - A rigorous review will detail every specimen-handling step, each decision point, and redundant activities - Task mapping can be applied to any segment of a laboratory’s workflow, whether technical or clerical WORKFLOW ANALYSIS - assimilates all of the previously discussed data and transforms them into valuable information - This should begin at or near the bedside to see how physicians are ordering tests and should proceed to specimen acquisition and delivery to the laboratory - A flow sheet, which follows the sample from initial order to arrival in the laboratory, should be created - Specimen transit through the laboratory should then be documented, noting areas where batch processing occurs - Using the sample arrival mapping done in data collection, an average time can be assigned by time of day. If this is done manually, it is best to select a number of key times and average them, if possible - Goal: Nonphysical bottlenecks should also be identified and quantified Workflow Modeling - they usually provide a somewhat static picture (i.e., each describes a single data element and often how it changes over time) - By using sophisticated workflow modeling software, one can analyze these complex interrelationships to better predict the outcome of a given workflow design Pneumatic Tube Transport of Specimens - can greatly decrease transport time and, thus, total turnaround time for test results - Once a laboratory has a tube system, it becomes very dependent on it, requiring a good service and support system to maintain it - Usually, the plant operations or engineering department of the hospital maintains the system on a daily basis UNDERSTANDING TECHNOLOGY - Laboratory technology - refers largely to 3 functional areas: - Testing equipment (i.e., analyzers) - Preanalytic processors - Information technology (IT) - the manner in which a laboratory information system is used for data retrieval and reporting (i.e., whether or not physicians directly access the LIS to view results) depends on whether a clinical information system is available to serve this purpose THE ROLE OF TECHNOLOGY - Technology: - is an integral part of a modern laboratory; however, it is not the solution to every problem - means to an end, not an end - Technology alone does not improve performance and workflow; it is only a tool to reach a goal - Knowing when to introduce a non-technologic solution instead of a technologic one can mean the difference between a targeted, cost-effective solution and an expensive one that does not fully address the initial problem, provides unnecessary functionality, or provides necessary functionality but at an unnecessary cost STRATEGIES TO OPTIMIZE PERFORMANCE STRATEGY Consolidate EXAMPLE One facility: Run stat and routine samples together on the same analyzer; run routine and specialty tests on the same platform; collapse number of analyzers and workstations and use workcell, if applicable. Consolidation can reduce “tube labor.” Multiple facilities: Centralize selected low-volume, high-cost tests/services at a single location (e.g., molecular diagnostics [HIV viral load], blood donor collection) Equipment: All equipment purchased from one vendor yields larger volume discounts and lower costs for reagents and analyzers, especially in chemistry and immunodiagnostics. Standardize Method: Uniform reference range for all laboratories promotes seamless testing environment for inpatients and outpatients with data comparability and trending results across laboratories. It also provides system backup without excess redundancy. billing), incremental testing costs, potential revenue, and core mission to determine whether to expand business Strategic sourcing Rapid repricing “Make versus buy” Policies: Simplify procedure manuals and compliance documents so they can be shared. Integrate Strategic growth management Evaluate excess capacity and feasibility of increasing testing workload: Assess outreach infrastructure (couriers, client/sales staff, with Review all send-out tests and low-volume in-house tests to identify which tests to “buy” (i.e., send out or outsource) and which to “make” (i.e., do in-house) based on cost, turnaround time, and/or clinical need. Also, review services, such as couriers. Can delta check limits be narrowed or eliminated to reduce the numbers of test repeats and verifications without compromising quality? is Computer: Network LIS with other data systems to promote seamless flow (e.g., sending point-ofcare results into the LIS). Courier: Use single service to deliver samples among multiple sites. Short-term strategy: Renegotiate pricing existing vendors Critically review laboratory policies and procedures to determine their relevance and appropriateness: Staff: Standardized operations make it easier to share staff among facilities. LIS: Database simplified. Long-term strategy: Competitively bid equipment, supplies, reference laboratory services, etc., taking into account payment terms, delivery charges, value-added services, and product costs. Review laboratory policies and tasks Do critical values need to be repeated before they are reported (Lehman et al., 2014)? Are critical call values clinically appropriate or do they generate unnecessary calls to physicians? Can nonurgent expensive tests be batched twice weekly instead of every day? Do clinicians need certain tests daily that are available only several times a week? Are quality control and maintenance procedures excessive? The rule-based autoverification process eliminates the need for Make maximum use the technologist to manually of simple and/or release each result (Crolla & existing IT solutions Westgard, 2003); sample racking storage system eliminates most of the time spent looking for samples. Cross-train staff Train technologists to perform automated chemistry and hematology tests instead of chemistry or hematology alone. Adjust skill mix Adjust skill level (and compensation) of staff to match task performed: Use laboratory helpers instead of technologists to centrifuge samples or load samples on analyzers. Adjust staff scheduling Use part-time phlebotomists to supplement peak blood collection periods instead of full-time phlebotomists who are underutilized once morning collection is finished. Change laboratory layout Manage utilization Design open laboratory that allows all automated testing to run in the same location and promotes cross-training of staff Require pathologist or director approval to order select costly reference laboratory tests and/or restrict usage of various tests to specialists LABORATORY MANAGEMENT Lesson 6: Pre-analysis Pre-analytic phase - defined as all the complex steps required before sample analysis - 1st and most crucial phase in the laboratory - major source of residual error Pre-analytic Factors - patient-related variables - specimen collection and labelling techniques - specimen preservatives and anticoagulants - specimen transport - specimen processing and storage Most Frequent Pre-analytic Errors - improperly filling the sample tube - placing specimens in the wrong container/preservatives - selecting the incorrect test Common Pre-analytical Errors (Specimen Collection) Pre-collection Variables A. Physiological factors 1. Diurnal Variation 2. Exercise - Transient effects: - free fatty acids – initial decrease then increase - lactate – increase 300% - CK, AST, LD – increase (*cardiac markers) - Activates coagulation, fibrinolysis, platelets - Long term effects: - CK, aldolase, AST, LD – increase - Prolactin – elevated - Serum gonadotropin – decrease 3. Diet - greatly affect lab results - transient, easily controlled - glucose, triglycerides, cholesterol, & electrolytes should be analyzed in the basal state - after 48 hours of fasting serum bilirubin is increased - after 72 hours of fasting plasma glucose is decreased while plasma triglycerides, glycerol, and free fatty acids are increased - long term vegetarians – decreased LDL, VLDL, total lipids, phospholipids, cholesterol, triglycerides, vitamin B12 - protein-rich diet – increased serum urea, ammonia, urate levels - high protein, low carb (Atkins diet) – increased serum BUN and ketones in urine - high unsaturated-to-saturated fatty acid ratio diet – decreased serum cholesterol - purine rich diet – increased urate value (nuts, munggo) - chronic alcohol abuse – elevated HDL, cholesterol, GGT, urate, MCV- (CBC) - obesity – increased LD, cortisol, glucose, cholesterol, triglycerides, apoB lipoproteins 4. Stress - Mental and Physical stress: increased ACTH, cortisol, catecholamines - Mild Stress: increased cholesterol, decreased HDL - Hyperventilation: acid base balance is affected, elevated leukocyte count, serum, lactate, free fatty acids 5. Posture - Elements affected by postural changes: - albumin - calcium - total protein - enzymes, triglycerides - bilirubin - cholesterol - Upright position: increases hydrostatic pressure, reduces plasma volume, and increased proteins - Supine position: elevated albumin and calcium Time of Collection - some samples have to be collected at a specific time - STAT (short turnaround time) - from Latin word: statim; immediately - the time period may vary from one lab to another - electrolytes, BUN, creatinine, AST, ALT, LDH, blood typing, and crossmatching are most commonly requested for STAT *cardiac markers* 6. Age - Men in 20s: uric acid at its peak - Post-menopausal women: increased in cholesterol because of decreased estrogen levels - Elderly men: decreased testosterone concentration - Elderly women: increased FSH Specimen Acceptability and Identification Issues - all specimens must be collected, labeled, transported, and processed according to established procedures - failure to follow specific procedures can result in specimen rejection - specimen rejection is costly and time-consuming - misidentification of patients is a life-threatening medical error - the 1st goal is to always identify patients correctly 7. Gender - Male (higher) - alkaline phosphatase - aminotransferase - creatine kinase - aldolase - Female (lower) - magnesium - calcium - albumin Reasons for Specimen Rejection - hemoglobin - serum iron - ferritin B. Tourniquet Application - using tourniquet for lactate concentration may result to falsely increased values - prolonged tourniquet application: may increase serum enzymes, proteins, cholesterol, calcium, triglycerides Specimen Collection Test Order - most frequent pre-analytic error involves wrong selection of lab tests - wrong laboratory tests/panel leads to inappropriate interpretation of results - official laboratory request is of utmost importance - patient demographics must be complete - lab information system (LIS) is used to generate requests - all specimens must be clearly labeled - add-on tests are tests requested on previously collected sample - policy on patient refusal - policy on difficulty of blood draw for some patients - policy on how to deal w/ combative patients - emergency measures for patients who become ill of faint during phlebotomy - the Philippine Data Privacy Act of 2012/RA 10173 Blood collection - Venipucture - performed using a needle/adapter assembly attached to an evacuated glass/plastic test tube w/ a rubber/plastic stopper - Syringe - helpful for procuring blood from hand/ankle/small children - Preferred site is median cubital vein Blood Collection Devices - syringes and needles (either made of plastic or glass) - needle gauge - winged infusion/butterfly (less painful for patients) - Evacuated tube (used when blood must be transferred faster before clot formation begins Other blood collection supplies - Tourniquet (helps to make veins more prominent) - Gloves - Gauze pads - Alcohol/iodine wipes Blood Collection Tubes Ethylene Diamine Tetraacetic Acid (EDTA) - anticoagulant of choice for hematology morphology - maybe spray-dried or liquid form Sodium Fluoride/Potassium Oxide - gray-stoppered tubes - used for glucose measurements - prevents glycolysis Sodium Citrate - light-blue stoppered tubes - preserves labile coagulation factors - used for coagulation studies - tube must be adequately filled Lithium Heparin/Sodium Heparin - green top tubes - useful for arterial blood gases - accelerate the action of antithrombin III, neutralizing thrombin, and prevents formation of fibrin Serum Separator Tubes - yellow top tubes - contain separation gel - used for routine chemistry tests Order of Draw Arterial Puncture - best done during steady state - should first do Modified Allen Test - preferred sites in order: radial, brachial, femoral arteries Finger and Heel Skin Puncture - routine assays requiring small amounts of blood - used for pediatric patients - in neonates: heel skin puncture (lateral plantar surface side of heel; warm (to avoid fluid build-up) - in older children: finger skin puncture Urine and Other Body Fluids Urine - laboratory testing of urine falls into 3 categories: - Chemical - Bacteriologic (macroscopic in other books) - Microscopic examinations (RBCs, AST, WBCs) Types of Urine Specimen Random - may be collected any time First morning - most concentrated; lower pH when asleep Midstream clean catch - for bacteriologic tests; 1st wipe genitalia then catch from the middle void not the first flow Timed - usually used for quantification of urobilinogen; peaks at 2 - 4 PM 24-hour - for creatinine clearances, collection of urine 24 hours in 4L sterile container then aliquot 40 mL for analysis Blood Collection Techniques Venipuncture - the blood is normally drawn from a vein on the top of the hand or from the inside of the elbow - patient must be in a seated or reclined position - median cubital vein (preferred site) - tourniquet must never be left longer than 1 minute; should be 3-4 inches above the proposed site Catheterized - either ureter or bladder catheters, used to detect infection from other kidney or bladder Suprapubic aspirate - directly aspirate urine from bladder (done by doctors) Other Body Fluids - Cerebrospinal Fluid (CSF) - routinely collected by lumbar puncture between the 3rd, 4th, or 5th lumbar vertebrae - to establish diagnosis of infection, malignancy, subarachnoid hemorrhage, multiple sclerosis or demyelinating disorders - Synovial fluid (joints) - Pleural fluid (lungs) - Pericardial fluid (around heart) - Peritoneal fluid (around stomach/gastric region) Specimen Transport and Processing Specimen Transport - for blood specimen, avoid excessive agitation to prevent hemolysis - for bilirubin determination, avoid light exposure - for ammonia, must be kept at 4C immediately after blood draw - for urine and other body fluids, all containers must be sterile and leak proof - labeling is of utmost importance in every specimen - when transporting specimens, biohazard logo is required “Infectious substance” - all lab specimen must be transported in a safe, convenient manner to prevent biohazard exposure or contamination of specimen Specimen Processing - ideally, specimens should be performed w/in 45 mins to 1 hour after collection - most blood specimens require centrifugation to separate the required samples (ex. Plasma, serum) - centrifuge uses centrifugal force to separate different phases of suspensions by different densities Interferences IN VIVO - tobacco smoking - increased cortisol, lactate, insulin - increased hemoglobin - decreased sperm counts & motility w/ abnormal morphology IN VITRO - collection-associated variables - hemolysis - light exposure - prolonged tourniquet application for lactate determination