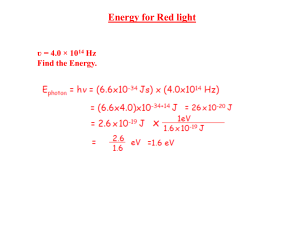
Trigger 5 Quantum Physics 1. Light shows wave properties such as reflection, refraction, diffraction and interference. (a) State which two of these properties best suggest that light is a wave rather than a particle. [1] (b) Describe how the dark positions in Young’s double-slit pattern can be explained if light is a wave, but cannot be explained if light is a series of particles. [2] 2. The wave theory tries to explain photoelectric emission as the slow absorption of energy by electrons from the wave, eventually giving them enough energy to escape. However, various observations in photoelectric emission suggest that light has particle properties, one of which is that there is a threshold frequency. (a) State what is meant by threshold frequency. [2] (b) Explain how it is difficult to explain a threshold frequency using wave theory. [2] (c) Explain how threshold frequency is explained in the particle theory, if the energy of the photon depends on frequency. [2] (d) State two other observations about photoelectric emission that suggest light has particle properties. [1] 3. (a) Explain the term photon. [2] (b) State what is meant by work function energy. [2] (c) Explain why the equation gives a maximum value for the kinetic energy of the emitted electron. [2] (d) Suggest why only a few electrons are emitted with the maximum kinetic energy. [2] Efendi Y., S.Si Page 1 Trigger 5 4. A beam of red light with the wavelength 750 nm is incident on a surface. The stopping voltage is V . The red light is replaced by violet light with the wavelength 375 nm. Estimate the stopping voltage now. [3] 5. (a) Outline what is meant by the de Broglie hypothesis. [2] (b) i. Show that the de Broglie wavelength of an electron that has been accelerated h from rest by a potential difference V is given by λ = √ 2mqV [2] ii. Calculate the de Broglie wavelength of an electron that has been accelerated from rest by a potential difference of 120 V. [1] (c) Outline an experiment in which the de Broglie hypothesis is tested. [3] (d) A bullet of mass 0.080 kg leaves a gun with speed 420 ms−1 . The gun is in perfect condition and has been fired by an expert marksman. The bullet must pass through a circular hole of diameter 5.0 cm on its way to its target. A student says that the bullet will miss its mark because of de Broglie’s hypothesis. By suitable calculations determine whether the student is correct. [4] Efendi Y., S.Si Page 2 Trigger 5 6. The theory predicts that the electron in the hydrogen atom has discrete or quantised energy. Show that the energy of an electron occupies the ground state is E = −13.6 eV. nh Hint: angular momentum is quantised mvr = 2π [8] 7. The figure below shows the lowest four energy levels of an electron in an isolated atom. Efendi Y., S.Si Page 3 Trigger 5 The figure below shows the lines in the emission spectrum of the atom that correspond to the transitions of the electron from n = 3 to n = 1 and from n = 4 to n = 1. (a) Explain, with reference to photons, why there is a single frequency of electromagnetic radiation that corresponds to each of these transitions. [2] i. On the second figure, draw a line that corresponds to the transition of the electron fron n = 2 to n = 1. Label this line A. [2] ii. On the second figure, draw a line that corresponds to the transition of the electron fron n = 3 to n = 2. Label this line B. [2] (b) The frequency of radiation represented by line A is fA . The frequency of radiation represented by line B is fB . The energy of the ground state (n = 1) is E1 . Determine an expression, in terms of fA , fB , E1 and the Planck constant h, for the energy E3 of the energy level n = 3. [2] Efendi Y., S.Si Page 4