Introduction to Bioorganic Chemistry and Chemical Biology
Answers to Chapter 6
(in-text & asterisked problems)
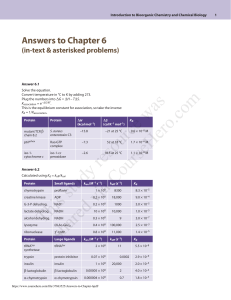
Answer 6.1
Protein
mutant TCR β
chain 8.2
p67phox
iso-1cytochrome c
Answer 6.2
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
Solve the equation.
Convert temperature in °C to K by adding 273.
Plug the numbers into ΔG = ΔH – TΔS.
Kassociation = e–ΔG/RT.
This is the equilibrium constant for association, so take the inverse:
Kd = 1/Kassociation.
Protein
ΔH
ΔS
(kcal mol–1)
(cal K–1 mol–1)
Kd
–15.8
–21 at 25 °C
9.6 × 10–8 M
Rac•GTP
complex
–7.3
52 at 18 °C
1.7 × 10–6 M
iso-1-cc
peroxidase
–2.6
18.5 at 25 °C
1.1 × 10–6 M
S. aureus
enterotoxin C3
Calculated using Kd = koff/kon:
Protein
chymotrypsin
Small ligands
proflavin
kon (M–1 s–1)
koff (s–1)
Kd
1 × 108
8300
8.3 × 10–5
ADP
0.2 × 108
18,000
9.0 × 10–4
G-3-P dehydrog.
NAD+
0.2 × 108
1000
5.0 × 10–5
lactate dehydrog.
NADH
10 × 108
10,000
1.0 × 10–5
alcohol dehydrog.
NADH
0.3 × 108
9
3.0 × 10–7
lysozyme
(N-Ac-Glu)2
0.4 × 108
100,000
2.5 × 10–3
3ʹ-UMP
0.8 × 108
11,000
1.4 × 10–5
Th
creatine kinase
ribonuclease
Protein
Large ligands
tRNASer
synthetase
tRNASer
trypsin
protein inhibitor
insulin
insulin
β-lactoglobulin
α-chymotrypsin
kon (M–1 s–1)
2 × 108
0.07 × 106
koff (s–1)
11
0.0002
Kd
5.5 × 10–8
2.9 × 10–9
1 × 108
20,000
2.0 × 10–4
β-lactoglobulin
0.00005 × 108
2
4.0 × 10–4
α-chymotrypsin
0.000004 × 108
0.7
1.8 × 10–3
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
1
2
Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 6
Answer 6.3
When the concentration of NADH is 3 × 10–7 M, the ratio of bound to unbound alcohol
dehydrogenase is 1:1. From there, the ratio of bound to unbound enzyme can easily be
estimated at other concentrations of NADH.
KD = 3
bound
enzyme•NADH
[NADH]
× 10–7 M
[enzyme•NADH]
unbound
enzyme
+
NADH
:
[enzyme]
:
1
M & Weiss | 978-0-8153-4214-4
1
:
3 ×Van
10–7
Vranken
1
3 × 10–6 M
10
Introduction to Bioorganic Chemistry and Chemical Biology | A6019
www.garlandscience.com
design
–8 M
1 by www.blink.biz
:
10
3 ש10
3 × 10–9 M
1
:
100
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
AWhen [NADH] = 3 μM, the ratio of bound to unbound enzyme is 10/11 ≈ 91%.
BWhen [NADH] = 3 nM, the ratio of bound to unbound enzyme is 1/101 ≈ 1%.
Answer 6.4
[Curcumin]
(µM)
O
OH
MeO
OMe
HO
OH
curcumin
5.5
11
55
550
5500
cancer cells
Dead / Live
Percentage
viable
1
2
10
100
1000
:
:
:
:
:
1
1
1
1
1
50
33
9.1
1.0
0.10
Introduction to Bioorganic Chemistry and Chemical Biology | A6117
Van Vranken & Weiss | 978-0-8153-4214-4
Curcumin has poor bioavailability. At an oral dose of 8
© www.garlandscience.com design by www.blink.biz
g of curcumin per day, the peak
serum concentrations of curcumin reach only 1.8 μM. Hypothetically, eating large
quantities of curcumin might be effective for colorectal cancer in the GI tract, but not for
systemic cancers like leukemias.
Answer 6.5
Galactose binds most tightly because it has the lowest Km; however, the affinities of
all three substrates are within a factor of two.
B
Galactose is isomerized more than 100 times faster than the other two substrates
on the basis of the kcat/Km values: galactose (3700 mM–1 s–1), glucose (13 mM–1 s–1),
xylose (20 mM–1 s–1).
C
If the system is at equilibrium, when the concentration of glucose is 10 Km (340 mM),
the ratio of glucose–enzyme complex to free enzyme will be 10:1. In a typical mammalian cell, the intracellular glucose concentration is less than 1 mM. Of course the
amount of free enzyme is likely to be small because galactose and other sugars can
occupy the enzyme active site.
Th
A
Answer 6.6
A
The substrate with the lowest Km binds most tightly: LRRASLG.
B
The substrate with the highest kcat is phosphorylated fastest (once it binds): LRRASLG.
C
The relative rates of phosphorylation will be proportional to kcat/Km. LRRASLG is better than LRAASLG by a factor of 1507.
Substrate
Km (μM)
LRAASLG
12200
8.7
0.00071
804
19.8
0.0246
31
33.1
1.07
LHRASLG
LRRASLG
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
kcat (s–1)
kcat / Km (M–1 s–1)
..
A
Introduction
toHBioorganic
Chemistry
and Chemical Biology: Answers to Chapter 6
OH
+H O
O
2
..
Ala NH2
Ala
Ala ..
N
H
OHC
N
H
OHC
Answer 6.7OHC
Ala +
N
H
Because imine formation is fast and reversible, the following mechanism is reasonable.
The mechanism for imine/iminium ion formation
was covered in Chapter
2.
OH
OH
Ala +
N
H
R SH
.. ..
Ala NH2
Ala
N..
HOH
Ala HO
..
N SR
H
O
O
OH OHC
Ala
R SH
..
R S
Ala
O
OH
SR
O
OH
Ala N
Ala
N:
R S H
+
SR
+
Ala N
Ala N
+
SR
Ala N
H
R S
R S
R S
: A-
The rate-determining step for this reaction has not yet been determined, making
it difficult to determine the ordering of the various steps. It has been proposed that
hemithioacetal formation precedes imine formation. Unfortunately, this proposed
mechanism involves the formation of a benzylic cation that is destabilized by the ortho
imine substituent.
unstable cation
R
+H
N
2O
R
+
R N
N
..
+
SCys
SCys
H
Cys S
Introduction to Bioorganic Chemistry and Chemical Biology | A6118
Van Vranken6.8
& Weiss | 978-0-8153-4214-4
Answer
www.garlandscience.com design by www.blink.biz
©
many
unstable cationprevent quantitative isolation of Asn, Gln,
Carboxamide
hydrolysis and β-elimination
steps
R Thr.
R
Ser, Cys, and
+
N
N
..
Asn
+H
O
2O
R N
+
NHSCys
2
O
NaOH
H
H
Cys S
SCys
N
N
-O
Introduction
to Bioorganic
100 °C Chemistry and Chemical Biology | A6118
H
Van Vranken & Weiss | 978-0-8153-4214-4
O
design byOwww.blink.biz
© www.garlandscience.com
NH2
Gln
O
O
O
N
H
HO
Cys
O
N
H
HS
Thr
O
H
N
H
H
N
H
H
N
H
O
NaOH
100 °C
H2N
Ser
O-
Th
N
H
H
N
+
SR
HO
N:
H
many
steps
N:
H
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
Ala
N
H
HO
..
OH
Ala +
N
H
+
OH
Ala N
Ala
HN
R S H : A+H2O
SR
OH
Ala N
Ala
N
H
+H2O
.. 2O
Ala +H
N
SR
H
OHC
H A
OHC
HO
N:
H
+
Ala
+ N
SRH
Ala
O
NaOH
100 °C
NaOH
100 °C
NaOH
NH2
-O
O
-O
O
N
H
HO
O
N
H
HS
O
H
-. N
.
O
N
H
H
N
O
O
-O
H
-. N
.
H
-. N
.
O
N
N
N
100 °C
H
H
H
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
HO
HO
Introduction to Bioorganic Chemistry and Chemical Biology | A6119
Van Vranken & Weiss | 978-0-8153-4214-4
www.garlandscience.com design by www.blink.biz
H
N
O
-O
O
3
4
Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 6
Answer 6.9
The enzyme uses two Zn2+ ions and an arginine to stabilize the serine alkoxide, the
alkoxide leaving group and the anionic phosphorane intermediate, but these are
omitted to simplify the problem.
Ser
- O:
Ser
O OP
O
-O
R
Ser
.. O OO
P
-O O
R
.. O OP
-O O
H
Ser
O
O
P
- O O - :O H
Ser
HOO
P
-O O- ORO
R
O
Ser
O
O- O OP
O
-O
H
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
Answer 6.10
Prostromelysin cannot cleave itself, because Cys75 holds the inhibitory domain in place
by coordinating to the Zn2+ ion at the active site (see the rendering of prostromelysin
in Figure 6.48). Arylmercurials have a high affinity for sulfur. They coordinate to Cys75,
opening up the Zn2+ active site, which can then proteolytically cleave the inhibitory
domain.
ArHg
Ar-Hg+
ArHg
S
S
S Zn
Zn
Zn
Introduction to Bioorganic Chemistry and Chemical Biology | A6120
Van Vranken & Weiss | 978-0-8153-4214-4
© www.garlandscience.com design by www.blink.biz
Answer 6.11
trapoxin peptide
Ph
Ph
O
O
NH HN
O
O
N H HN
N
H
OH
Zn2+ binding element
Th
O
Introduction to Bioorganic Chemistry and Chemical Biology | A6121
Van
Vranken & Weiss
| 978-0-8153-4214-4
Introduction
to Bioorganic
Chemistry and Chemical Biology | A6122
www.garlandscience.com
design by www.blink.biz
©Van
Vranken & Weiss | 978-0-8153-4214-4
Answer
6.12
design by www.blink.biz
© www.garlandscience.com
If the two ligands bound with perfect cooperativity, the dissociation constant would be
the product of the two Kd values, namely 10–6 × 10–6 = 10–12 M.
Answer 6.13
Bn
+
N
:
- S
Me
H
S
O
R
Bn
+
N
:
- S
Me
R
B H
Me
- .. Bn
O
N
R
+
B:
Bn
+
N
Me
S
R
CO2Me
B:
H
H Bn
O
+
N
S
CO2Me
Me
R
CO2Me
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
Me
H
+
BnN
Me
R
H B
+
BnN
R
Bn
+
N
:
- S
- .. Bn
O
N
Me
R
H Bn
O
+
N
S
Me
S
CO2Me
Me
+
BnN
HO
Me
S
H
R Chemical Biology: Answers to Chapter 6
IntroductionRto Bioorganic Chemistry and
CO2Me
H Bn
O
+
N
-..
B:
Me
+
O
CO2Me
Me
R
S
5
H B
.. CO2Me
R
+
BnN
H O
B:
S
CO2Me
R
CO2Me
Me
R
+
BnN
- ..O
S
Bn
+
N
:
- S
O
CO2Me
CO2Me +
Me
R
Introduction to Bioorganic Chemistry and Chemical Biology | A6123
Van
Vranken6.14
& Weiss | 978-0-8153-4214-4
Answer
© www.garlandscience.com design by www.blink.biz
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
O
Enz
NH2
HO3PO
H2N:
NH2
O
Enz
O
O-
N
HO3PO
OH
Me
N+
H
O-
+
H N
H
B:
HO3PO
Me
HO3PO
O-
OH
N+
H
OH
Me
N+
H
Enz
O
: NH
O-
:
N
H2N H
+
N
N+
H
O
Enz
OH
Me
N
H2N:
H
HO3PO
OH
Enz
O
Enz
O-
CO2-
HN
NH
HO3PO
O-
Me
+ N
H
Me
Introduction to Bioorganic Chemistry and Chemical Biology | A6124
Van Vranken & Weiss | 978-0-8153-4214-4
Answer
6.15
www.garlandscience.com
design by www.blink.biz
©
A
({G/C}{A/C}T)6
B
{G/A}{T/C}G{G/C}{G/C}G{G/T}{T/C}G{G/T}{T/C}G{G/A}{A/C}G
C
{G/C}{C/A}C{G/C}CGG{C/T}G{G/T}CGG{C/A}G
*Answer 6.16
Chorismate binds more tightly because it has the lower Km.
B
Chorismate is also rearranged more quickly (after it binds) because it has the much
larger kcat.
Th
A
COverall, chorismate (kcat/Km = 207 mM–1 s–1) is a better substrate than the O-methyl
analog (kcat/Km = 0.29 mM–1 s–1) by almost three orders of magnitude.
*Answer 6.17
A
O
H
N
OH
O
O
strained
reactive
intermediate
Me
B
OH
OH
O
O
H
H
HN Enz
HN Enz
N
N
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
O
O
O
O
..
O
O
O +H N
O +H N
3
3
Me
Me
H
O
H
N
OH..
OO
Me
HN Enz
O
+H
3N
O
O
H
N
OH
O
O
Me
A
O
6
OH
H
N
O
strained
reactive
intermediate
O
Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 6
Me
B
OH
O
H
N
O
HN Enz
O
Me
B:
O
H
+H
O
O
3N
OH
O
H
N
O
..
O
-
Me
HN Enz
+H
O
O
3N
OH..
O-
H
N
HN Enz
O
O
+H
Me
3N
OH
H
N
O
O
O
O
Me
C
O
OH
H
N
O
H
HN Enz
:O
O
+H
O
O
3N
HN Enz
O
+H
O
O
3N
OH
O
H
N
Me
O
.. -
H
O
O
O
3N
HN Enz
O
+H
O
H
3N
O
non-fluorescent
O
Cl
Me
Me
O+
R
N
H
+H
OH
O
H
N
Me
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
..
N
H
HN Enz
O
Cl
D
R
O
H
Cl
O
OH
..
O-
H
N
O
..
H2N
O
O
+
H2N
O
fluorescent
O
O-
Lone pair donation of the amino group into the coumarin ring system favors a cross-conjugated, non-aromatic form. When
Introduction to Bioorganic Chemistry and Chemical Biology | A6125
aminocoumarin
is conjugated to a peptide as an amide, the amino lone pair donates more into the amide carbonyl than into the
Van Vranken & Weiss | 978-0-8153-4214-4
© www.garlandscience.com
coumarin
ring system.design by www.blink.biz
*Answer 6.20
A
- Enz
S
..
HO2C
AcAspGluVal
H
N
H
O
HO2C
N
H
AcAspGluVal
N
N
H
O..
AcAspGluVal
naphth
N
H
H B
CbzAspGluVal
N
H
Enz
S
OH
Enz
HO2C
N
O
HO2C
Enz
S
- Enz
S
..
O
B
CbzAspGluVal
HO2C
S
N
..
N
O
naphth
Enz
HO2C
O
naphth
CbzAspGluVal
N
H
S
O
N
N
O
naphth
H B
C
- Enz
S
..
O
O
HO2C
N
Th
CbzAspGluVal
HO2C
N
H
O
N
Bn
Bn
CbzAspGluVal
Ser
O
N
:O
R
Enz
O
N
O
O
.. -
HO2C
N
Bn
H B
Introduction to Bioorganic Chemistry and Chemical Biology | A6128
Van Vranken & Weiss | 978-0-8153-4214-4
*Answer
6.22
www.garlandscience.com
design by www.blink.biz
©
O
N
H
S
Ser
- O:
N
Ser
O
O
O
O
R
NH
R
O
Introduction to Bioorganic Chemistry and Chemical Biology | A6130
Van Vranken & Weiss | 978-0-8153-4214-4
© www.garlandscience.com design by www.blink.biz
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
Bn
CbzAspGluVal
N
H
S
Enz
O
N
O
OH
N
Bn
Bn
naphth
naphth
Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 6
*Answer 6.25
The wild-type enzyme processes aspartate 1.2 × 106 times faster. The R292D variant
exhibits reversed selectivity, favoring arginine. However, the R292 variant processes
arginine more than about 104 times slower than the wild-type enzyme processes
aspartate.
NH2
NH2
H
N
O
N
H +
O
D223
NH2
H
N
K258
HO3PO
O
-
O
NH2
-O
OH
D292
O
+
+H
2N
R386
N
H
Introduction to Bioorganic Chemistry and Chemical Biology | A6133
Van Vranken & Weiss | 978-0-8153-4214-4
*Answer
6.27
www.garlandscience.com
design by www.blink.biz
©
OH
O
H
N
S
O
O
H
C6H13
B:
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
Cinnabaramide A is a strained β-lactone, structurally similar to salinosporamide. The
nucleophilic threonine of the proteasome reversibly attacks the β-lactone.
O
NHAc
CO2Me
OH
H
N
O
O
O
H
C6H13
OH
O
H
N
S
..
O
-
C6H13
H
N
CO2Me
HN Enz
:O
+H
O
C6H13
OH
O
H
N
OH
C6H13
S
NHAc
CO2Me
HN Enz
O
O
O
3N
O
NHAc
OH..
O-
+H
3N
O
Introduction to Bioorganic Chemistry and Chemical Biology | A6134
Van Vranken & Weiss | 978-0-8153-4214-4
*Answer
6.28
© www.garlandscience.com design by www.blink.biz
A
H
H
O
O H N
N
N
G
N H N
N
H
H
N
N
H
H
O H N
N
N
N H O
C
N
G
N H
N
N
N
N
A
O
Introduction to Bioorganic Chemistry and Chemical Biology | A6135
Van Vranken & Weiss | 978-0-8153-4214-4
www.garlandscience.com
design
by www.blink.biz
©Although
some of the
codons
below are susceptible to
Th
B
H
mutations would be silent.
Lys
(AAA, AAG)
Met
(AUG)
Glu
(GAA, GAG)
Gly
(GGU, GGC, GGA, GGG)
Trp
(UGG)
Ile
(AUU, AUC, AUA)
Val
(GUU, GUC, GUA, GUG)
STOP (UAG, UGA, UAA)
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
C to A transversion, those
7
8
Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 6
*Answer 6.30
sh is
ar stu
ed d
vi y re
aC s
o
ou urc
rs e
eH w
er as
o.
co
m
One way to approach this problem would be to examine each of the five histidine
residues in neuropsin to see which one is close to an Asp and a Ser residue. The catalytic
triad involves residues Asp57, His102, and Ser195.
Th
Introduction to Bioorganic Chemistry and Chemical Biology | A6137
Van Vranken & Weiss | 978-0-8153-4214-4
© www.garlandscience.com design by www.blink.biz
https://www.coursehero.com/file/37863525/Answers-to-Chapter-6pdf/
Powered by TCPDF (www.tcpdf.org)