The structure of metals
M. Vedani
Applied Metallurgy - AY 2022/23
Disclaimer: The content of this document and related video of the lecture is intended only for personal use of students
attending the course of “Applied Metallurgy” held at Politecnico di Milano in the AY 2021/22. Any content generated
out of it cannot be reproduced (as a whole or in part), stored, transmitted or published on any other website by the
Users, nor are the Users allowed to create any derivative work based on this material without the written permission of
the author.
A common metal «sample» observed at different
magnifications
A common metal «sample» observed at different
magnifications
Still increasing magnification …
The crystal structure
A crystal is defined as an orderly array of atoms in space
The unit cell of a crystal structure is the smallest group of atoms having the symmetry
of the crystal which, when repeated in all directions, can develop the crystal lattice
The 14 types of Bravais lattices
There exist in nature 14 types of lattices grouped in 7 crystal systems
The body centered cubic (BCC) cell
Most metals crystallize in one of these three structures: BCC, FCC, HCP
Examples of BCC Metals: a-Fe, Cr, Nb, V
2 atoms per unit cell
APF = Natoms·Vatoms / Vunit cell
APF (atomic packing factor): 0,680
The face centered cubic (FCC) cell
Examples of FCC Metals: g-Fe, Al, Cu, Au, Ni
4 atoms per unit cell
APF : 0,741
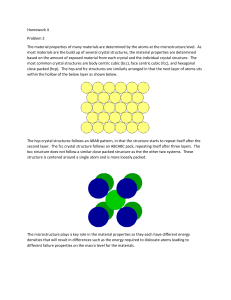
The hexagonal close packed (HCP) cell
Examples of HCP Metals: a-Ti, Mg, Zn, Co
6 atoms per unit cell
APF: 0,741
The atomic packing factor (APF)
APF is is the fraction of volume in a crystal structure that is occupied by constituent
atoms:
APF = Natoms·Vatoms / Vunit cell
APFBCC= 0.68
APFFCC= 0,74
APFHCP= 0,74
A comparison between the FCC and the HCP structure shows that they are quite
similar, with the only difference in the packing sequence:
A-B-A-B for HCP, A-B-C-A-B-C for FCC
Effects of crystal orientation
✓ The spacing among atoms is not always the same in all crystallographic directions
✓ Most properties change according to crystallographic directions in crystal lattices
✓ A preferred texture might develop in metals after specific treatments or manufacturing
processes
The Miller indexing system for directions in cubic crystals
For directions:
✓ specific directions: m: [111] n: [101]
✓ family of directions m: <111>
For plane identification in cubic crystals:
✓ specific planes: (100)
✓ family of planes: {100}
For hexagonal structures, a four-digit indexing system is used to facilitate
planes/direction identification
Crystal bonds
Crystalline solids are grouped into four classifications:
✓ Ionic;
✓ Van der Waals;
✓ Covalent
✓ Metallic
The internal energy of a crystal is composed of two parts:
✓ the lattice energy U: the potential energy due to electrostatic attractions and
repulsions that atoms exert on one another
✓ the thermal energy of the crystal associated to the vibrations of the atoms about
their equilibrium positions
To make things simpler, we can suppose to operate at zero absolute temperature so as
to neglect the contribution of the thermal energy
Crystal bonds
Considering for simplicity a ionic bond (e.g. NaCl)
the Coulomb force attracting a pair of ions at a distance r and the corresponding
potential energy are:
F=
k 1 e1 e 2
r12 2
k e e
U(r ) = Fdr = 1 1 2
r12
r
short range force also acts, generating a repulsions when the atmospheres of the two
atoms begin to overlap
k e e
k e e
U(r ) = Ftot dr = − 1 1 2 + 2 1 2
r12
r n
r
12
N A z 2 e2 N B e2
U( r ) = U0 −
+
r
r9
Crystal bonds
Metallic bonds
✓ metallic bond forms when atoms give out their valence electrons, which then
form an electron sea
✓ The positively charged atom cores are bonded by mutual attraction to the
negatively charged electrons