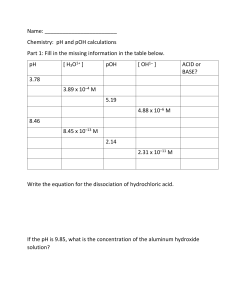
p6 U5 O View feedback Play All View hints Question 1 8n 6U W bb قيَم ـُة pHلَم حلوٍل ماٍّيئ ِع نَد درجـِة َح رارِة الُغ رفـِة ُت ساوي .8.09 َس يكوُن يف َه ذا الَم حلوِل 1 . 23 × 10 −6 M t.m Question 2 . e/+ C ] [ OH − https://t.me/+CbbW8n6Up6U5OGE8 اْكُت ِب الَّر قَم الَّص حيَح َم َع َم ِزن َلَت ِني َع شرَّي َت ِني يف الَف راِغ الُم خَّص ِص ِل َت كتمَل ا ُجل مَل ـُة . p6 U5 O اْس َح ِب ا ِإل جاباِت الَّص حيَح ـَة إىل ا َأل ماكِن الُم ناسَب ـِة ِل َي كَت مَل ا َجل دوُل ِب رَت اكِزي االِّت زاِن . ) H + (aq َت ركُزي ِّت اال زاِن () M 0.01 0 8n 6U 0 0.01 35 0.625 W Question 3 e/+ C bb 0.635 0 https://t.me/+CbbW8n6Up6U5OGE8 الرَّت كُزي االبتداُّيئ () M HCOO − (aq ) + ⇌ ) H (aq t.m p6 U5 O اخِرت ا ِإل جاَب ـَة الَّص حيَح ـَة ِم َن ا َخل ياراِت الواردِة َأ دناُه . 8n 6U الَم حلوُل َ 1أ كُرث َح مضَّي ـًة ِم َن الَم حلوِل .2 ] [ OH − ] [H + َأ كُرب يف الَم حلوِل .1 t.m Question 4 e/+ C َأ كُرب يف الَم حلوِل .1 bb W الَم حلوُل َ 2أ كُرث قاِع دَّي ـًة ِم َن الَم حلوِل .1 https://t.me/+CbbW8n6Up6U5OGE8 باْس ِت خداِم ِم قياِس َ ،pHت َّم قياُس pHللَم حلوَل ِني 1و َ ،2و ُو ِج َد َأ َّن ِق َيَم َ pHل ُه ما 8.56و 6.56على الَّت وايل. اخِرت الِع بارَة الَّص حيَح ـَة َع ِن الَم حلوَل ِني 1و .2 p6 U5 O اْكُت ِب الَّر قَم الَّص حيَح َم َع َم ِزن َلَت ِني َع شرَّي َت ِني يف الَف راِغ الُم خَّص ِص ِل َت كتمَل ا ُجل مَل ـُة . ِع نَد درجـِة َح رارِة الُغ رفـِة K w = [ OH − ][H + ] = 1 . 00 × 10 − 14 ِم ْن ِخ الِل َت طبيِق سالِب الُّل وغاريتِم ِلِك ال جانِّيب َت ع ِريب Kw : 8n 6U 273 K K w = 1 . 14 × 10 − 15 ، َس َي كوُن ناُجت ُ pH + pOHي ساوي 14 . 94 . Question 5 اْد ُر ِس الُم عادَل ـَة الكيميائَّي ـَة ِل َت أُّي ِن َح مٍض َض عيٍف يف َم حلوٍل ماٍّيئ : ) A − (aq HA (aq ) ⇌ H + (aq ) + e/+ C bb W 273 K ِع نَد درجـِة َح رارِة https://t.me/+CbbW8n6Up6U5OGE8 َ ،س َت كوُن الَع الَق ـُة َب َني pHو pOHعلى الَّن ح َّت ِو ِع نَد درجـِة َح رارِة ال ايلpH + pOH=14 : اْس َح ِب ا ِإل جاباِت الَّص حيَح ـَة إىل ا َأل ماكِن الُم ناسَب ـِة ِل كتاَب ـِة َت عبِري ثابِت َت َأ ُّي ِن ا َحل مِض ِب َش كٍل َص حيٍح . t.m p6 U5 O 8n 6U .2 2 االتزان ] − [H +االبتدائي ] [ HA Question 6 e/+ C bb W اخِرت ا ِإل جاَب ـَة الَّص حيَح ـَة ِم َن ا َخل ياراِت الواردِة َأ دناُه . t.m اْس ُت خدَم ِم قياُس ِ pHل قياِس pHالْثَن ِم َن الَم حالي المائَّي ـِة الُم حَّض رِة ِم ْن َح ْم َض َض عيَف رَت اكَزي ِل ِني ِني ِني ِب ُم َت ساوَي ـٍة . ُو ِج َد َأ َّن pHللَم حلوِل ُ 1ي ساوي ،2.00و pHللَم حلوِل ُ 2ي ساوي .3.00 https://t.me/+CbbW8n6Up6U5OGE8 .1 االتزان [H + ] 2 1 p6 U5 O اخِرت الِع بارَة الَّص حيَح ـَة َح وَل ثاِب ِت َت َأ ُّي ِن ا َحل ْم ِض للَم حاليِل 1و .2 K aللَم حلولِ K a = 2للَم حلولِ 1 K aللَم حلولِ K a < 2للَم حلولِ 1 8n 6U Ka للَم حلوِل على قيَم ـِة .pH K aللَم حلولِ K a > 2للَم حلولِ 1 W bb Question 7 e/+ C اْكُت ِب الَّر قَم الَّص حيَح َم َع َم ِزن َلَت ِني َع شرَّي َت ِني يف الَف راِغ الُم خَّص ِص ِل َت كتمَل ا ُجل مَل ـُة . https://t.me/+CbbW8n6Up6U5OGE8 ال َي عتمُد t.m p6 U5 O َم حلوُل َح مِض الَه يدروكلوريِك ذو قيمـة .pH = 3.50 َس َي كوُن الرَّت كُزي االبتداُّيئ َحِل مِض الَه يدروكلوريِك 3 . 16 × 10 −4 M . Question 8 8n 6U رك يوناِت الَه يدروكسيِد ا يت ُي نِت ُج ها Sr ( OH ) 2 قاعدِة َه يدروكسيِد الّس رتونشيوِم ( ) الَق وَّي ـِة وَت ركُزي ها 0 . 016 M M 0.032 َأ َق ُّل ِب َك ثٍري ِم ْن ُي ساوي َأ َق ُّل ِب َك ثٍري ِم ْن 0.016 0.016 0.032 M M M e/+ C Question 9 bb . W ُي ساوي t.m اْكُت ِب الَّر قَم الَّص حيَح َم َع َم ِزن َلَت ِني َع شرَّي َت ِني يف الَف راِغ الُم خَّص ِص ِل َت كتمَل ا ُجل مَل ـُة . https://t.me/+CbbW8n6Up6U5OGE8 َتاْس ُزيَح َأ ِب ا ِإل جاَب ـَة الَّص َّلحيَح ـَة إىلَتَفالُّكَم ُككاِن الُم ناسِب ِل َت كتمَل ا ُجل مَل ـُة . p6 U5 O َم حلوٌل ماٌّيئ َت ركُزي َأ يوِن الَه يدروجِني فيه 0 . 012 M 1.92 8n 6U Question 10 Drag and drop the CORRECT options to complete the table with the equilibrium concentrations. initial concentration (M ) 0.625 Question 11 HCOO − (aq ) bb 0.635 e/+ C equilibrium concentration (M ) ⇌ 0.635 + 0 W HCOOH (aq ) t.m https://t.me/+CbbW8n6Up6U5OGE8 . . لهذا الَم حلوِل الماِّيئpH َس َت كوُن قيَم ـُة 0.01 0 By taking the negative logarithm of both sides of Kw K w = 1 . 14 × 10 − 15 pH + pOH will be equal to 14 . 94 8n 6U At 273 K: at 273 K. W Question 12 e/+ C bb . اْكُت ِب ا َأل رقاَم الَّص حيَح ـَة َم َع َم ِزن َلَت ِني َع شِر َّي َت ِني يف الَف راغاِت الُم خَّص صـِة : يف َه ذا الَم حلوِل. ِع نَد درجـِة َح رارِة الُغ رفـِة،pH =9.10 َم حلوٌل ماٌّيئ َل ُه t.m https://t.me/+CbbW8n6Up6U5OGE8 expression, pH and pOH will be related as follows: pH + pOH = 14.00 p6 U5 O Fill in the blank with the CORRECT number with two decimal places. At room temperature: K w = [ OH − ][H + ] = 1 . 00 × 10 − 14 [H + ] 7 . 94 × 10 − 10 M = 4.90 = pOH Question 13 p6 U5 O اخِرت ا ِإل جاباِت الَّص حيَح ـَة ِم َن الَق وائِم الُم نسدَل ـِة ِل َت كتمَل ا ُجل مُل . الَم حلوُل ذو قيَم ـة pOH = 5هو َ 10,000م َّر ة َأ كرَث قاِع دَّي ـًة َت ركُزي ِم ْن َم حلوٍل له .pOH = 9 ] [H + 8n 6U Question 14 يف َم حلوٍل ماٍّيئ . Question 15 اخِرت ا ِإل جاباِت َأالَّص حيَح ـَة ِم َن الَق وائِم الُم نسدَل ـِة ِل َت كتمَل ا ُجل مُل . إذا كاَن ت pHكَرب ِم ْن ِ 7ع نَد درجـِة َح رارِة الُغ رفـِة َ ،س َت كوُن قيَم ـُة pOH t.m َأ َق َّل ِم ْن .7 e/+ C bb W اخِرت ا ِإل جابـَة الَّص حيَح ـَة ِم َن القاِئ َم ـِة الُم نسدَل ـِة ِل َت كتمَل ا ُجل مَل ـُة . pOHهو ساِل ُب ُل وغاريتِم َت ركِزي َأ يوناِت الَه يدروكس ِدي https://t.me/+CbbW8n6Up6U5OGE8 يف َم حلوٍل ذي قيَم ـة ُ pOH = 5ي ساوي 1 . 00 × 10 −9 M . p6 U5 O ُيَع ُّد الَم حلوُل الماُّيئ ذو قيَم ـِة َ pHأ كَرب ِم ْن 7قاِع دًّي ا . Question 16 8n 6U ِم قياُس pH ُت ُب ر قاُّيل امليثيِل e/+ C bb َو َر َق ـُة َت َّب ِعا الَّش مِس W َأ وراُق كاِش ِف pH Question 17 https://t.me/+CbbW8n6Up6U5OGE8 َأاخِرت ا ِإل جاَب ـَة الَّص حيَح ـَة ِم َن ا َخل ياراِت الواردِة َأ دناُه . ٌّي ِم َّم ا يلي ُيَع ُّد َط ريَق ـًة َد قيَق ـًة ِل َت حديِد قيَم ـِة pHللَم حلوِل ؟ t.m p6 U5 O 8n 6U Which of the following is true about pure water at any temperature? pOH = 7 W [H + ] = [ OH − ] pH + pOH = 14.00 bb pH = 7 e/+ C https://t.me/+CbbW8n6Up6U5OGE8 Select the CORRECT answer. Question 18 t.m Fill in the blank with the CORRECT numbers with two decimal places. The pH of an aqueous solution at room temperature is 9.10. For this solution: [H + ] pOH = p6 U5 O 7 . 94 × 10 − 10 = M 4.90 8n 6U Select the CORRECT choice from the drop-down menus. 10,000 times more basic pOH = 9. The solution with pOH = 5 will have + [H ] 1 . 00 × 10 −9 Question 20 M. bb = than a solution with W A solution with pOH = 5 is e/+ C https://t.me/+CbbW8n6Up6U5OGE8 Question 19 Select the CORRECT choice from the drop-down menu. pOH is the negative logarithm of the t.m aqueous solution. hydroxide ions concentration in an Fill in the blank with the CORRECT number with two decimal places. An aqueous solution has a hydrogen ion concentration of 0.012M. 1.92 8n 6U . Question 22 Select the CORRECT choice from the drop-down menus. W At room temperature when the pH is larger than 7, the pOH will be 7. bb An aqueous solution with such pH is considered to be basic smaller than . e/+ C https://t.me/+CbbW8n6Up6U5OGE8 The pH of this aqueous solution will be p6 U5 O Question 21 Question 23 Drag and drop the CORRECT option to complete the sentence. 0.016M of the strong base strontium hydroxide ( t.m Sr ( OH ) 2 ) produces hydroxide ions with a concentration equal to 0.032 M. much smaller than 0.032 8n 6U pH meter litmus paper methyl orange bb universal indicator strip t.m Question 25 W Select the CORRECT answer. Which of the following is an accurate method to determine a solution's pH? e/+ C https://t.me/+CbbW8n6Up6U5OGE8 equal to 0.016 Question 24 p6 U5 O much smaller than 0.016 The initial concentration of the hydrochloric acid would be e/+ C Question 26 bb W M. p6 U5 O 8n 6U A solution of hydrochloric acid has a pH = 3.50. t.m https://t.me/+CbbW8n6Up6U5OGE8 Fill in the blank with the CORRECT numbers with two decimal places. 3 . 16 × 10 −4 p6 U5 O 8n 6U The [ OH − ] 1 . 23 × 10 −6 bb in this solution will be equal to W The pH of an aqueous solution was measured and found to be 8.09 at room temperature. e/+ C https://t.me/+CbbW8n6Up6U5OGE8 Fill in the blank with the CORRECT number with two decimal places. M. Question 27 Consider the chemical equation for the ionization of a weak acid in an aqueous solution: + − HA (aq ) ⇌ H (aq ) + A (aq ) t.m Drag and drop the options to correctly write the acid ionization constant expression. equilibrium [ HA ] initial − [H + ] 2 equilibrium W Question 28 p6 U5 O [H ] 1 8n 6U 2. + 2 e/+ C bb Select the CORRECT choice. A pH meter was used to measure the pH of two aqueous solutions prepared from two weak acids t.m https://t.me/+CbbW8n6Up6U5OGE8 1. of equal concentrations. The pH of solution 1 was found to be 2.00 and that of solution 2 was found to be 3.00. Choose the correct statement about the acid ionization constant for solutions 1 and 2. Ka is independent of a solution's pH bb e/+ C Select the CORRECT answer. W Question 29 8n 6U K a of solution 1 > K a of solution 2 t.m https://t.me/+CbbW8n6Up6U5OGE8 K a of solution 1 = K a of solution 2 p6 U5 O K a of solution 1 < K a of solution 2 Using a pH meter, the pH of solutions 1 and 2 was measured and found to be 8.56 and 6.56 respectively. Choose the correct statement about solutions 1 and 2. p6 U5 O [ OH − ] is larger in solution 1. [H + ] e/+ C bb W is larger in solution 1. 8n 6U Solution 2 is more basic than solution 1. t.m https://t.me/+CbbW8n6Up6U5OGE8 Solution 1 is more acidic than solution 2.