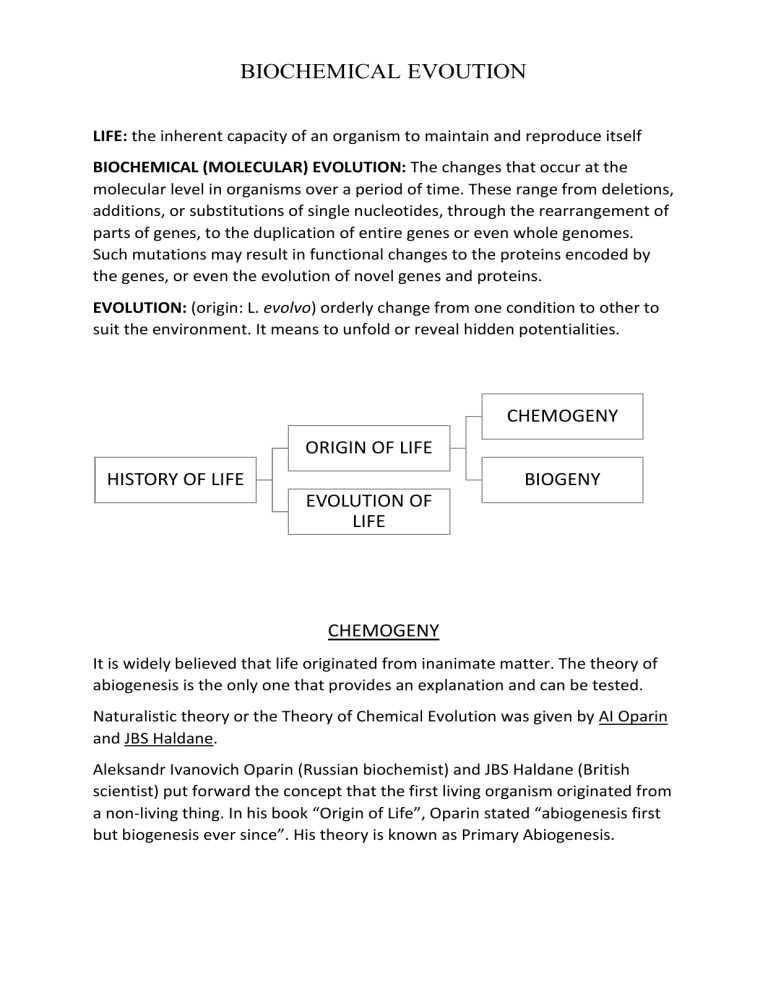
BIOCHEMICAL EVOUTION LIFE: the inherent capacity of an organism to maintain and reproduce itself BIOCHEMICAL (MOLECULAR) EVOLUTION: The changes that occur at the molecular level in organisms over a period of time. These range from deletions, additions, or substitutions of single nucleotides, through the rearrangement of parts of genes, to the duplication of entire genes or even whole genomes. Such mutations may result in functional changes to the proteins encoded by the genes, or even the evolution of novel genes and proteins. EVOLUTION: (origin: L. evolvo) orderly change from one condition to other to suit the environment. It means to unfold or reveal hidden potentialities. CHEMOGENY ORIGIN OF LIFE HISTORY OF LIFE BIOGENY EVOLUTION OF LIFE CHEMOGENY It is widely believed that life originated from inanimate matter. The theory of abiogenesis is the only one that provides an explanation and can be tested. Naturalistic theory or the Theory of Chemical Evolution was given by AI Oparin and JBS Haldane. Aleksandr Ivanovich Oparin (Russian biochemist) and JBS Haldane (British scientist) put forward the concept that the first living organism originated from a non-living thing. In his book “Origin of Life”, Oparin stated “abiogenesis first but biogenesis ever since”. His theory is known as Primary Abiogenesis. Oparin and Haldane suggested what the sequence of events might’ve been. The chemical evolution is divided into four phases: I. ATOMIC PHASE Early Earth consisted of elements essential for the formation of the protoplasm (hydrogen, oxygen, carbon, nitrogen, sulphur, phosphorus, etc.). Atoms were segregated in three concentric masses based on their weights: • Heaviest elements like iron, nickel and copper were found in the centre • Medium weight elements like sodium, potassium, chlorine and fluorine were found in the outer core • Lightest elements like nitrogen, oxygen and hydrogen found in the primitive atmosphere II. ORIGIN OF MOLECULES AND INORGANIC COMPOUNDS Free atoms combine to form molecules and simple inorganic compounds. Hydrogen atoms are most reactive and numerous in primitive atmosphere. Primitive atmosphere was a reducing atmosphere as H atoms first combined with all O atoms to form H2O, leaving no free oxygen. H atoms also combined with N to form NH3. Thus, water and ammonia were the first compound molecules of primitive earth. III. ORIGIN OF SIMPLE ORGANIC COMPOUNDS The primitive atmosphere contained gases like CO2, CO, H2, N2, etc. nitrogen and carbon in the atmosphere combined with metallic atom forming nitrides and carbides. Water vapors and metallic carbide reacted to form the first organic compound – CH4. Further, HCN was formed. Torrential rains might’ve caused water to rush down that dissolved and carried salts and minerals with it to accumulate in oceans. The early compounds reacted and produced simple organic compounds like simple sugars (ribose, deoxyribose, glucose), nitrogen bases (purines, pyrimidines), amino acids, glycerol, fatty acids) Some external sources such as solar radiations, energy from electrical discharges like lightening, high energy radiation must have provided energy for the reactions. There was no ozone layer in the atmosphere. The oceanic water rich in mixture of organic compounds was termed as “hot dilute soup” or prebiotic soup by JBS Haldane. Thus, the stage was set for combination of various chemical elements. Once formed, they accumulated in water because their degradation was slow in the absence of catalysts. IV. ORIGIN OF COMPLEX ORGANIC COMPOUNDS A variety of amino acids, fatty acids, hydrocarbons, purine and pyrimidine bases and simple sugars are present in ancient sea. Electrical discharge, lightening, solar energy, ATP and polyphosphates might have provided the source of energy for polymerization reactions of organic synthesis. Thus, small simple organic molecules combined to form large complexes. • Amino acids combined to form polypeptides and proteins • Simple sugars combined to form polysaccharides • Fatty acids and glycerols combined to form fats i. ii. iii. iv. v. vi. CO2 – CO + [O] CO2 + 2[O] – CH2O + H2O CO +NH3 – HCN + H2O CH4 + NH3 – HCN + 3H2 HCN + NH3 + CH2O – NH2-CH2-CN + H2O NH2-CH2-CN + 2H2O – NH2-CH2-COOH + NH3 MILLER’S EXPERIMENT BIOGENY CONDITIONS FOR ORIGIN OF LIFE • A supply of replicators or self-producing molecules • Mutations during the copying of replicators (high temperature must have played a major role) • Continuous supply of free energy and partial isolation from the general environment. ORIGIN OF PREBIOTIC MOLECULES Partial isolation has been attained with aggregates of artificially formed prebiotic molecules. These aggregates are called protobionts, which can separate combinations of molecules from the surroundings. They maintain an internal environment but are unable to reproduce. Two important protobionts are: Coacervates. Oparin observed that if a mixture of large proteins and a polysaccharide is shaken, coacervates are formed. They show simple form of metabolism and as they don’t have lipid outer membranes, they cannot reproduce. Microspheres. When mixtures of artificially produced organic compounds are mixed with coal water, microspheres are formed. Sydney Fox heated a mixture of 18 amino acids at 130 - 180°C and obtained stable proteins like macromolecules, which he named proteinoids. STRUCTURAL PROPERTIES OF PROTENOID MICROSPHERES Under an electron microscope, concentric double layered boundaries around them have been observed through which diffusion of material occurs. They have the ability of motility, growth, binary fission and a capacity of reproduction by budding and fragmentation. EVOLUTION OF ENZYME SYSTEM Enzymes that control the types and rates of reactions within organisms are proteins. These proteins are synthesized by a process that begins with the transcription of information from DNA to mRNA. rRNA has catalytic properties along with informational properties, due to which the first genetic code was based on RNA catalyzed its own replication as well as catalyzing other chemical reactions. The accumulated products of RNAcatalyzed reaction and form structures. For example, RNA could have catalyzed the formation of lipid-like molecule that could have in turn formed plasma membrane and proteins. Further, this protein could have been successful to catalyze the synthesis of ither proteins. Eventually, the proteins might have taken over most enzymatic functions because they are better catalysts than RNA and are capable of more diverse specific activities. If the first cells used RNA as their hereditary molecule, which later evolved into DNA. DNA probably did not evolve as a hereditary molecule until RNA-based life became enclosed in membrane. Once cells evolved, DNA probably replaced RNA as genetic code for most organisms. GENETIC REGULATION MECHANISMS In cells of body, all genes not active all the time. A gene became active only when the product of the genes required by the cell. It means a mechanism controls the rate of polypeptide synthesis, amount of polypeptide and time at which a gene should be active and which a gene should be inactive. This control over polypeptide synthesis is known as gene regulation. It is studied with the help of operons. GENE REGULATION IN PROKARYOTES Lactose operon in E.coli Operon synthesis process was proposed by Jacob and Monad. R P O Z Y A Operon. It is a part of genetic material which acts as a single regulated unit having one or more structural genes – a regulator gene(R), a promoter gene(P), an operator gene(O), a repressor and an inducer or compressor (structural genes). Operons are of two types – inducible and repressible. INDUCIBLE OPERON Switches on in response to the presence of a chemical. It consists of the parts: 1. Structural Genes: synthesize mRNA. An mRNA controls metabolic activity of cytoplasm through the formation of protein or enzymes over the ribosomes. An operon can have one or more structural genes lac-operon has 3- Z,Y,A. They transcribe polycistronic mRNA molecules that helps in synthesis of three enzymes: • β-galactosidase. For hydrolysing lactose to galactose • Lactose or galactose permease. For allowing the entry of lactose from outside the cell • Galactose acetylase or transacetylase. Helps the bacteria in utilisation of glucose by bacteria 2. Operator Genes: directly controls the synthesis of mRNA. Pragati Dhingra | 03916001319