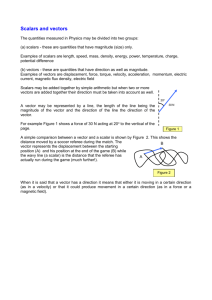
0. Physical quantities and SI units Subtopics 1. 2. 3. 4. Physical quantities SI units Errors and uncertainties Scalars and vectors 0.1 Physical quantities Our goals: 1. 2. Understand that all physical quantities consist of a numerical magnitude and a unit make reasonable estimate of physical quantities included within the syllabus 0.1 Physical quantity definition: a feature of something which can be measured, for example, length, weight, or time. Consist of a magnitude and a unit. Example of physical quantity: magnitude unit 0.1 Physical quantities questions: 1. 2. estimate the mass of an apple estimate the volume of a basketball 0.2 SI units Our goals: 1. 2. 3. 4. recall the following SI base quantities and their units: mass (kg), length (m), time (s), current (A), temperature (K) express derived units as products or quotients of the SI base units and use the derived units for quantities listed in this syllabus as appropriate use SI base units to check the homogeneity of physical equations recall and use the following prefixes and their symbols to indicate decimal submultiples or multiples of both base and derived units: pico (p), nano (n), micro (μ), milli (m), centi (c), deci (d), kilo (k), mega (M), giga (G), tera (T) 0.2 SI units Base units Derive units definiton : seven fundamental SI definition : product of base units example of some derive units 0.2 SI units Determine derive units: 1. 2. write down the formula of a quantity write each base unit from the formula example: determine the base units of speed? 0.2 SI units Determine the units of the following quantities: 1. Density 2. Pressure 0.2 SI units Homogenity of units means each term has the same base units. example : unit of u and at have the same unit as v. question: show that the left hand side of the equation : pressure + ½ x density x speed^2 = constant is homogeneous and find the base units of the constant on the right hand side 0.2 SI units Prefixes 0.3 Errors and uncertainties Our goals: 1. 2. 3. understand and explain the effects of systematic errors (including zero errors) and random errors in measurements understand the distinction between precision and accuracy assess the uncertainty in a derived quantity by simple addition of absolute or percentage uncertainties 0.3 Error and uncertainties Systematic error : all readings being either above or below the accepted value and cannot eliminated by repeating reading and then averaging. Examples of systematic errors: 1. zero error on an instrument 2. 3. wrongly caliberated scale reaction time of experimenter Random error : readings being scattered around the accepted value, may be reduced by repeating a reading and averaging, and by plotting a graph and drawing a best-fit line. examples: 1. 2. reading a scale from different angles Accuracy and precision Uncertainties: 1. Absolute uncertainty : absolute uncertainty 2. percentage uncertainty : Combining uncertainties: 1. If formula is addition or substraction, absolute uncertainty of each quantity added, example: 2. If formula is multiplication or divisor, percentage uncertainty of each quantity added, example: Percentage uncertainty of P Absolute uncertainty of P 3. If formula is power/root, percentage uncertainty multiply by factor of power of the quantity, example: Define absolute uncertainty of with: 0.4 Scalars and vectors Our goals: 1. 2. 3. understand the difference between scalar and vector quantities and give examples of scalar and vector quantities included in the syllabus add and subtract coplanar vectors represent a vector as two perpendicular components 0.4 Scalars and vectors Scalar : a quantity which can be describe fully by giving its magnitude. Vector: a quantity which has magnitude and direction 0.4 Scalars and vectors Vector Representation 0.4 Scalars and vectors Addition of vector : connects vectors 0.4 Scalar and vectors Resolution of vectors