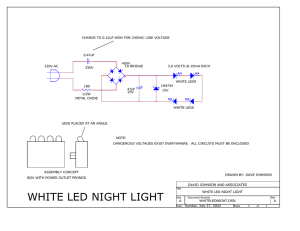
The LED Development of LEDs V G Harris 10/19/2022 Fundamentals A light-emitting diode (LED) is a semiconductor light source that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresponding to the energy of the photons) is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device https://en.wikipedia.org/ wiki/Light-emitting_diode https://warwick.ac.uk/fac/sci/physics/current/postgraduate/re gs/mpagswarwick/ex5/devices/led/ 1st Generation Appearing as practical electronic components in 1962, the earliest LEDs emitted low-intensity infrared (IR) light. Infrared LEDs are used in remote-control circuits, such as those used with a wide variety of consumer electronics. The first visible-light LEDs were of low intensity and limited to red. Early LEDs were often used as indicator lamps, replacing small incandescent bulbs, and in seven-segment displays. Later developments produced LEDs available in visible, ultraviolet (UV), and infrared wavelengths, with high, low, or intermediate light output, for instance white LEDs suitable for room and outdoor area lighting. LEDs have also given rise to new types of displays and sensors, while their high switching rates are useful in advanced communications technology. https://en.wikipedia.org/wiki/Light-emitting_diode Blue LED (controversy) In the late 1980s, key breakthroughs in GaN epitaxial growth and p-type doping ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, Theodore Moustakas at Boston University. Two years later, in 1993, high-brightness blue LEDs were demonstrated by Shuji Nakamura of Nichia Corporation using a gallium nitride growth process. In parallel, Isamu Akasaki and Hiroshi Amano of Nagoya University were working on developing the important GaN deposition on sapphire substrates and the demonstration of ptype doping of GaN. This new development revolutionized LED lighting, making high-power blue light sources practical, leading to the development of technologies like Blu-ray. Nakamura, Hiroshi Amano and Isamu Akasaki were awarded the Nobel Prize in Physics in 2014 for the invention of the blue LED. In 2015, a US court ruled that three companies had infringed Moustakas's prior patent, and ordered them to pay licensing fees of not less than US$13 million. https://en.wikipedia.org/wiki/Light-emitting_diode 1.6 x 1.6 x 0.35 mm3 The Edison light bulb converts electrical energy into heat! (thousands of filaments!) However, electrical energy can also be converted to light! The LED! • Electrical energy flowing through it is directly converted into light energy! • Light is made up of many small particles called photons. Photons have energy and momentum but no mass. • What is Light Emitting Diode (LED)? • Recall: • Photon is a Boson - Like the God-like Higgs Boson • Fermion is a particle that obeys Fermi exclusion principle – like and electron….etc LEDS are the most popular of semiconductor pn diodes…. • LEDS emit visible light or IR in forward bias (consider laser pointers versus IR remote controls) • LEDS are an optical semiconductor pn diode that converts electrical energy to visible light • In pn forward bias – electrons recombine with holes and emit a photon- called electroluminescence Normal pn junction • To create an LED, the n-type material should be connected to the negative terminal of the battery and p-type material should be connected to the positive terminal of the battery. In other words, the n-type material should be negatively charged and the p-type material should be positively charged. • The construction of LED is similar to the normal p-n junction diode except that gallium, phosphorus and arsenic materials are used for construction instead of silicon or germanium materials Heat generation vs light generation • In normal p-n junction diodes, silicon is most widely used because it is less sensitive to temperature. Also, it allows electric current efficiently without any damage. In some cases, germanium is used for constructing diodes. • However, silicon or germanium diodes do not emit energy in the form of light. Instead, they emit energy in the form of heat. Thus, silicon or germanium is not used for constructing LEDs. Layers of LED • A Light Emitting Diode (LED) consists of three layers: p-type semiconductor, n-type semiconductor and depletion layer. The p-type semiconductor and the n-type semiconductor are separated by a depletion region or depletion layer. P-type semiconductor • When trivalent impurities are added to the intrinsic or pure semiconductor, a p-type semiconductor is formed. • In p-type semiconductor, holes are the majority charge carriers and free electrons are the minority charge carriers. Thus, holes carry most of the electric current in p-type semiconductor. N-type semiconductor • When pentavalent impurities are added to the intrinsic semiconductor, an n-type semiconductor is formed. • In n-type semiconductor, free electrons are the majority charge carriers and holes are the minority charge carriers. Thus, free electrons carry most of the electric current in n-type semiconductor. Depletion layer or region • Depletion region is a region present between the p-type and n-type semiconductor where no mobile charge carriers (free electrons and holes) are present. This region acts as barrier to the electric current. It opposes flow of electrons from n-type semiconductor and flow of holes from p-type semiconductor. How Light Emitting Diode (LED) works? • Light Emitting Diode (LED) works only in forward bias condition. When Light Emitting Diode (LED) is forward biased, the free electrons from n-side and the holes from p-side are pushed towards the junction. • When free electrons reach the junction or depletion region, some of the free electrons recombine with the holes in the positive ions. We know that positive ions have less number of electrons than protons. Therefore, they are ready to accept electrons. Thus, free electrons recombine with holes in the depletion region. In the similar way, holes from p-side recombine with electrons in the depletion region. Recombination….the devil is at play! • Because of the recombination of free electrons and holes in the depletion region, the width of depletion region decreases. As a result, more charge carriers will cross the p-n junction. • Some of the charge carriers from p-side and n-side will cross the p-n junction before they recombine in the depletion region. For example, some free electrons from n-type semiconductor cross the p-n junction and recombines with holes in p-type semiconductor. In the similar way, holes from p-type semiconductor cross the p-n junction and recombines with free electrons in the n-type semiconductor. • Thus, recombination takes place in depletion region as well as in p-type and n-type semiconductor. • The free electrons in the conduction band releases energy in the form of light before they recombine with holes in the valence band. • In silicon and germanium diodes, most of the energy is released in the form of heat and emitted light is too small. • However, in materials like gallium arsenide and gallium phosphide the emitted photons have sufficient energy to produce intense visible light. How LED emits light? • When external voltage is applied to the valence electrons, they gain sufficient energy and breaks the bonding with the parent atom. The valence electrons which breaks bonding with the parent atom are called free electrons. • The energy level of all the valence electrons is almost same. • The energy level of free electrons in the conduction band is high compared to the energy level of valence electrons or holes in the valence band. Therefore, free electrons in the conduction band need to lose energy in order to recombine with the holes in the valence band. • The free electrons in the conduction band do not stay for long period. After a short period, the free electrons lose energy in the form of light and recombine with the holes in the valence band. Each recombination of charge carrier will emit some light energy • The brightness of the emitted light depends on the material used for constructing LED and forward current flow through the LED. • In normal silicon diodes, the energy gap between conduction band and valence band is less. Hence, the electrons fall only a short distance. As a result, low energy photons are released. These low energy photons have low frequency which is invisible to human eye. • In LEDs, the energy gap between conduction band and valence band is very large so the free electrons in LEDs have greater energy than the free electrons in silicon diodes. Hence, the free electrons fall to a large distance. As a result, high energy photons are released. These high energy photons have high frequency which is visible to human eye. The efficiency of generation of light in LED • The efficiency of generation of light in LED increases with increase in injected current • In light emitting diodes, light is produced due to recombination process. Recombination of charge carriers takes place only under forward bias condition. Hence, LEDs operate only in forward bias condition. • When light emitting diode is reverse biased, the free electrons (majority carriers) from n-side and holes (majority carriers) from p-side moves away from the junction. As a result, the width of depletion region increases and no recombination of charge carriers occur. Thus, no light is produced. • All diodes emit photons or light but not all diodes emit visible light. The material in an LED is selected in such a way that the wavelength of the released photons falls within the visible portion of the light spectrum. • Light emitting diodes can be switched ON and OFF at a very fast speed of 1 ns. Direct-bandgap materials make better LEDs than indirect bandgap materials. Direct-bandgap materials make better LEDs than indirect bandgap materials. LEDs Solar cells Performance: Haitz's law Liu, Zongyuan & Liu, Sheng & Wang, Kai & Luo, Xiaobing. (2009). Status and prospects for phosphor-based white LED packaging. Frontiers of Optoelectronics in China. 2. 119-140. 10.1007/s12200-009-0011-2. Different colors! • LEDs are available in different colors. The most common colors of LEDs are orange, yellow, green and red. Indium gallium nitride (InGaN, InxGa1−xN) is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared (0.69 eV) for InN to the ultraviolet (3.4 eV) of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7. White LEDs: RGB vs Phosphors • There are two primary ways of producing white light-emitting diodes. • One is to use individual LEDs that emit three primary colors—red, green and blue—and then mix all the colors to form white light. • The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, similar to a fluorescent lamp. The yellow phosphor is cerium-doped YAG crystals suspended in the package or coated on the LED. • This YAG phosphor causes white LEDs to appear yellow when off, and the space between the crystals allow some blue light to pass through in LEDs with partial phosphor conversion. In LEDs with PFS phosphor, some blue light passes through the phosphors, the Ce:YAG phosphor converts blue light to green and red (yellow) light, and the PFS phosphor converts blue light to red light. • The best color rendition LEDs use a mix of phosphors, resulting in less efficiency and better color rendering. • The first white light-emitting diodes (LEDs) were offered for sale in the autumn of 1996. https://en.wikipedia.org/wiki/Light-emitting_diode https://en.wikipedia.org/wiki/Light-emitting_diode White LED Usually blue InGaN LEDs coated with yellow phosphor epoxy encapsulation- cerium-doped yttrium aluminium garnet (Ce3+:YAG) Blue light emitted by InGaN hits the coating which emits yellow light. Blue Light + Yellow Light = “White” Light In terms of wavelength, (460-490 nm) + (500-700 nm) Using several phosphor layers of distinct colors broadens the emitted spectrum, effectively raising the color rendering index (CRI) broad band emission of YAG:Ce phosphor originates from the 5d→4f transition of Ce3+ *PL : Photoluminescence PL emission spectra of (Y 1–xCex)3Al5O 12 phosphors sintered What determines the color of an LED? • The material used for constructing LED determines its color. In other words, the wavelength or color of the emitted light depends on the forbidden gap or energy gap of the material. • Different materials emit different colors of light. • Gallium arsenide LEDs emit red and infrared light. • Gallium nitride LEDs emit bright blue light. • Yttrium aluminium garnet LEDs emit white light. • Gallium phosphide LEDs emit red, yellow and green light. • Aluminium gallium nitride LEDs emit ultraviolet light. • Aluminum gallium phosphide LEDs emit green light. More on color and efficiency Bright LEDs – obnoxious headlights! The first white LEDs were expensive and inefficient. However, the light output of LEDs has increased exponentially, with a doubling occurring approximately every 36 months since the 1960s (similar to Moore's law). This trend is generally attributed to the parallel development of other semiconductor technologies and advances in optics and materials science and has been called Haitz's law after Dr. Roland Haitz. LED construction One of the methods used to construct LED is to deposit three semiconductor layers on the substrate. The three semiconductor layers deposited on the substrate are n-type semiconductor, ptype semiconductor and active region. Active region is present in between the ntype and p-type semiconductor layers. Advantages of LED • The brightness of light emitted by LED is depends on the current flowing through the LED. Hence, the brightness of LED can be easily controlled by varying the current. This makes possible to operate LED displays under different ambient lighting conditions. • Light emitting diodes consume low energy. • LEDs are very cheap and readily available. • LEDs are light in weight. • Smaller size. • LEDs have longer lifetime. • LEDs operates very fast. They can be turned on and off in very less time. • LEDs do not contain toxic material like mercury which is used in fluorescent lamps. • LEDs can emit different colors of light. Disadvantages of LED • LEDs need more power to operate than normal p-n junction diodes. • Luminous efficiency of LEDs is low. Applications of LED • • • • • • • • • • • • The various applications of LEDs are as follows Burglar alarms systems Calculators Picture phones Traffic signals Digital computers Multimeters Microprocessors Digital watches Automotive heat lamps Camera flashes Aviation lighting White LED Usually blue InGaN LEDs coated with yellow phosphor epoxy encapsulation- cerium-doped yttrium aluminium garnet (Ce3+:YAG) Blue light emitted by InGaN hits the coating which emits yellow light. Blue Light + Yellow Light = “White” Light In terms of wavelength, (460-490 nm) + (500-700 nm) Using several phosphor layers of distinct colors broadens the emitted spectrum, effectively raising the color rendering index (CRI) broad band emission of YAG:Ce phosphor originates from the 5d→4f transition of Ce3+ *PL : Photoluminescence PL emission spectra of (Y 1–xCex)3Al5O 12 phosphors sintered fini